[1021] Tissue Engineering and Organ Regeneration
In the rapidly evolving field of regenerative medicine, the convergence of cellular technology, gene editing, and bioengineering presents unparalleled opportunities to develop groundbreaking therapies. Our research group is dedicated to bridging the gap between basic research and clinical applications, with a particular focus on pioneering treatments for Type 1 diabetes.
Overcoming the Challenges of Islet Transplantation
Islet transplantation, a promising treatment for Type 1 diabetes, faces several challenges. Right after transplantation, many islets are lost due to an instant inflammatory reaction, destroying up to 70% of the islets. Poor blood supply to the islets leads to oxygen and nutrient shortages, causing cell death.
In addition to these immediate threats, transplanted islets face ongoing challenges over time. The liver, where the islets are typically transplanted, lacks the natural pancreatic environment, which makes it difficult for the islets to function properly, leading to long-term deterioration.
To overcome these obstacles, we are exploring innovative bioengineering approaches to develop alternative sources of endocrine cells and create technologies that mimic the natural islet environment.
Our Integrated Strategies
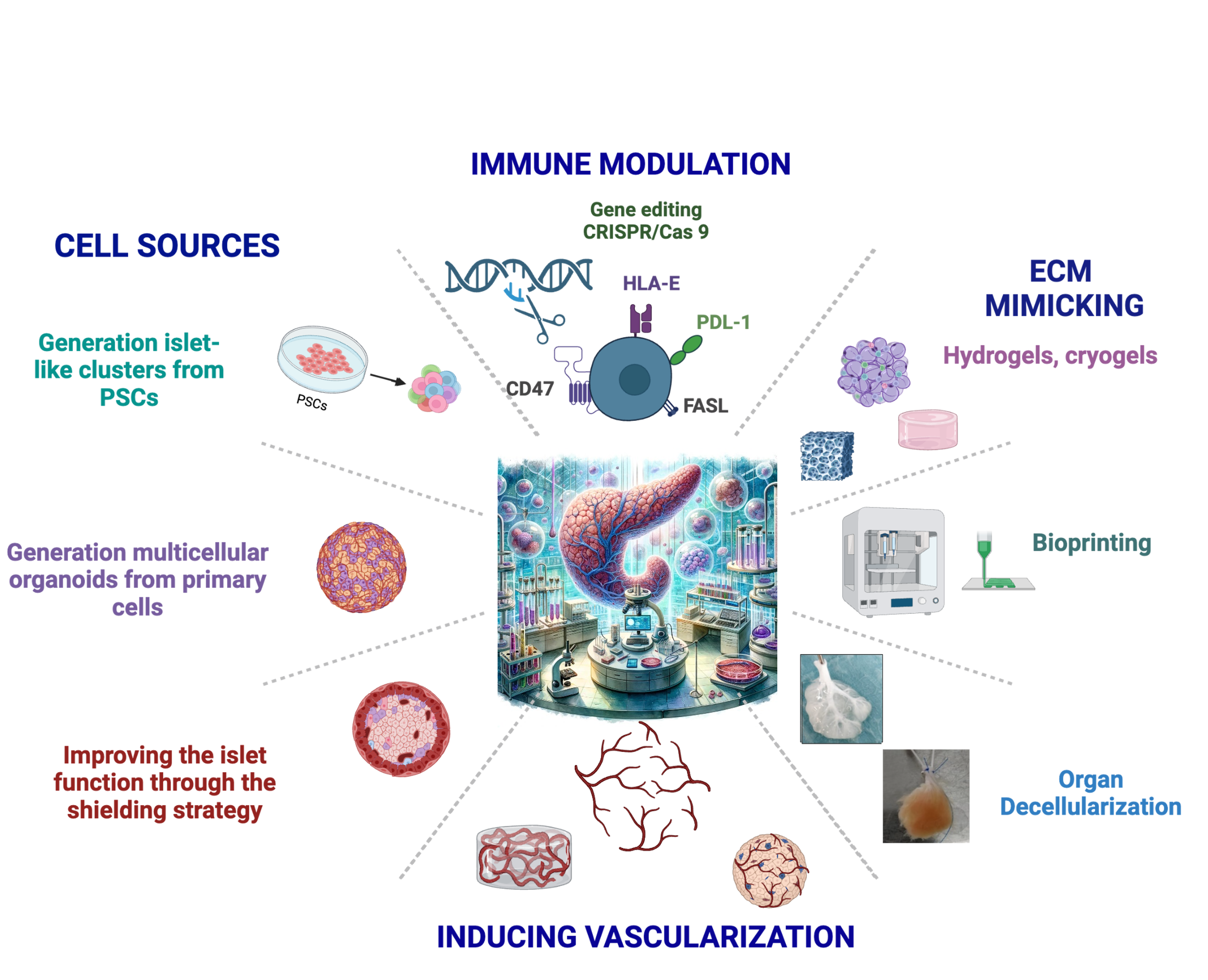
We are committed to developing an Advanced Therapy Medicinal Product (ATMP) to cure Type 1 diabetes. To achieve this ambitious goal, we are pursuing multiple interconnected strategies:
Generation of Insulin-Producing Cells and Organoids
- Stem Cell Differentiation: Developing infinite sources of insulin-producing cells derived from induced pluripotent stem cells (iPSCs) and human embryonic stem cells (hESCs).
- 3D Organoid Technology: Employing 3D organoid generation to create functional islet-like structures that closely mimic native pancreatic islets.
Bioengineered Scaffolds and Biomaterials
- Innovative Scaffold Design: To recreate the native islet microenvironment and improve biocompatibility, we generate scaffolds from fetal matrices, which serve as the foundation for our prevascularized hydrogel and cryogel platforms. These engineered scaffolds simulate the natural pancreatic environment, promoting cell survival, functionality, and integration by delivering essential extracellular matrix components, as well as oxygen and nutrients, while supporting the formation of functional vasculature within the scaffold.
- Decellularization Techniques: We employ advanced organ decellularization methods to remove cellular material while preserving the extracellular matrix (ECM) structure. This retains essential biochemical and mechanical cues, promoting vascularization and integration of insulin-producing cells. By maintaining the ECM's architecture, these scaffolds enhance cell adhesion, proliferation, and function, supporting long-term regenerative outcomes
Vascularization Strategies
Effective vascularization is essential for the survival and function of transplanted tissues. We specialize in bioengineering functional microvascular networks to address this challenge:
- Bioengineering Microvascular Networks: We integrate recipient-derived human endothelial cells into biocompatible hydrogels, cryogels, and scaffolds. These constructs form organized vascular networks that, when implanted into animal models, reconnect with the host vasculature, facilitating robust blood flow.
- In Vivo Vascular Integration: Our model is well-suited for studying the cellular and molecular mechanisms of vascular network formation and provides a platform for developing new strategies to engineer vascularized tissues.
- Endothelial-Stem Cell Interactions: This approach allows us to explore how endothelial cells modulate the behavior of co-transplanted stem cells, and how tissue-specific endothelial cells influence the communication between stem cells and the vasculature.
- Enhancing Engraftment and Function: By promoting the development of rich vascular networks within our scaffolds, we ensure the supply of essential extracellular matrix (ECM) components, oxygen, and nutrients, ultimately enhancing cell survival, maturation, and functionality, including insulin secretion.
Immune Modulation and Tolerance Induction
- Immune-Modulating Scaffolds: Our approach focuses on integrating immune-modulating molecules and immunoregulatory cells directly into biocompatible scaffolds.
- Immune Cloaking Strategies: We engineer insulin-secreting cells and accessory cells with advanced immune cloaking techniques.
Our ultimate aim is to develop a sustainable, long-term cure for Type 1 diabetes. By generating functional insulin-producing cells, recreating the natural islet environment with advanced biomaterials, and inducing immune tolerance, we strive to overcome current limitations in islet transplantation.





