EBM, Education & Health Services Research
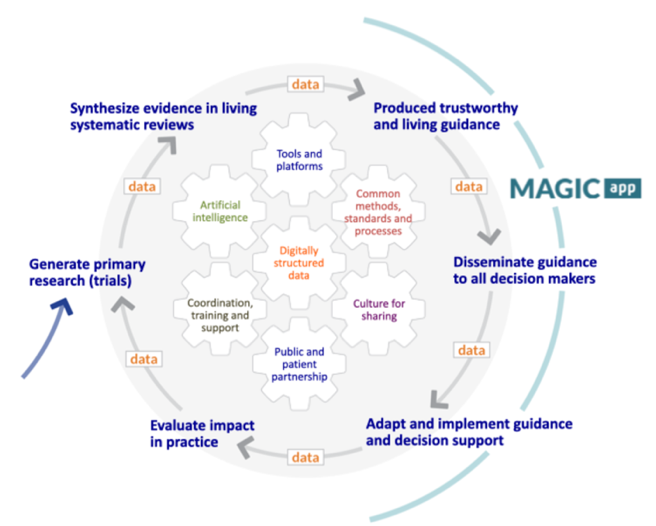
The group conducts research on knowledge translation, and implementation to enhance evidence-based practice througout each step of the “Evidence Ecosystem” (see Figure). This involves research on evidence synthesis, critical appraisal, guideline methodology and dissemination formats, access and use at the point of care, patient partnership, and shared decision making. Further down the implementation loop, it involves research in medical education, and health services research in our acute-care wards.
Examples of our work across the evidence ecosystem includes the following:
- Evidence production: The group develops methods for study design and conduct of current trials & cohort studies in the division and institution.
- Evidence synthesis and appraisal: We lead and contribute to numerous systematic reviews and meta-analysis. Prof Agoritsas contributes to methodological developments in network meta-analysis (NMA) and Living evidence and guidelines. He is an active member of the GRADE working group (www.gradeworkinggroup.org), issuing methodological guidance. He also contributes to JAMA Users’ Guide on core methodological issues (e.g. meta-analysis, prognosis, statistical adjustment, subgroup analyses). Prof Jean-Luc Reny, MD, PhD, and Dr Chrisophe Marti, MD, CC, contribute to meta-analyses, notably on thrombosis and pulmonary embolism.
- Guidelines methodology: Prof Agoritsas is leading since 2015 the BMJ Rapid Recommendations (www.bmj.com/rapid-recommendations), an international collaboration with the British Medical Journal to identify practice changing evidence through structured monitoring, conduct rapid systematic reviews on the topic, gather an unconflicted guideline panel in patient parternship, to issue a recommendation using GRADE and translate them in online multilayered formats. Since COVID-19 outbreak, this led to an ongoing Living NMA and methodological support of WHO Living Guidance on drugs for COVID-19. The group has also been involved in coordinating a task forces authoring local guidelines at the University hospitals of Geneva, disseminating them online.
- Shared Decision Making and Patient partnership: The group leads SHARE-IT and MATCH-IT projects, developing and testing generic digital tools to support shared decision making at the clinical encounter. These are implemented in an online authoring and dissemination plateform – MAGICapp (www.magicevidence.org/magicapp/) – to explore how they can be linked to digitally structured guidelines, evidence summaries. Patients parternship is central to these developments and the group is a major contributor in the institutions’ patients parternship programme. Prof Agoritsas also co-chairs the Association Savoir Patient (https://savoirpatient.ch) building key collaboration since 2012 with the University of Geneva.
- Medical education is central in enhancing knowledge transfer into evidence based practice. Prof Mathieu Nendaz, MD, MHPE, and Dr Matteo Coen, MD, PhD, are leading research on the mecansims underpinning medical reasoning (includind bias), learning and evaluation, communication, and inter-professional interventions to encance knowledge transfer and evidence based practice. Prof Nendaz has been for many years partly in the SMIG and partly attached to UDREM (50-50). He is the director of UDREM since 2015, while keeping 30% activity in the SMIG. Dr. Coen, also has a 30% activity in UDREM.
- Implementation and evaluation of health services: Next down the loop of the evidence ecosystem comes the implementation of evidence and evaluation of practice, both locally in our acute wards, and in contribution to internationally funded projects:
§ Locally, Prof Agoritsas and Dr Darbellay have been instrumental in the design and implementation of the institutionnal program “More time for patients” aimed at simplifying clinical and administrative processes using tools from lean management and design thinking. Dr Clément Bluclin, our MD-PhD candidate co-supervised by Prof Agoritsas & Prof Courvoisier, is conducting the formal evaluation of this program, both retrospectively, including data on >10’000 inpatients, and prospectively in a Cluster Randomized Trial assessing the boosting of implementation intervention in 20 hospital units (ClinicalTrial.gov: NCT06491797). He is also leading the largest Systematic Review on interventions that enhance patient-dedicated time in clinical encounters (117 studies, comprising alsmot 300’000 patients), as well as contributing to key other assessement or services. Dr Nils Burgisser has also developed expertise clinical registries through our electronic health records (EHR). He is establishing an automated, self-updating gout patent registry, eliminating the need for manual recruitment and data collecton, and includes large language models (LLM) to improve disease detecton in EHR free- text data. He is now developing a collaboration with Prof Fahad Razak leading the GEMINI research team at the University of in Toronto, Canada, a unique research databases, holding relevant clinical and administrative data from over 2.4 million hospital admissions over more than a decade. In our group, Dr Darbellay, MD is also leading the “Nightshift project”, an ecological study evaluationg medical calls in general internal medicine wards that included 40 physicians, and directly observed 66 different shifts over 6 months, allowing 1051 individual interventions to be analyzed. Dr Marti is conducting the evaluating the role and outcomes of our Intermedidate Care unit, particularly throughout the COVID-19 pandemic in relationship with the guidelines developed by this group.
§ Internationnally, our group is leading or contributing to several large scale implementation project through MAGIC Ecosystem Foundation (full list can be found on: https://magicevidence.org/research-projects/). For instance, BE-SAFE (Horizon Europe 2022-2027; https://besafe-horizon.eu) aims to improve patient safety and quality of care through patient-centred and evidence-based interventions to reduce benzodiazepine and sedative hypnotic use. The Global Evidence, Local Adaptation project (GELA, Horizon Europe 2022-2025; https://africa.cochrane.org/projects/GELA) aims to the impact of research on poverty-related diseases through a programme of improving decision makers’ capacity to use global research to develop locally relevant guidelines for newborn and child health in three sub-Saharan African countries. The Optimising Colorectal Cancer Prevention Trough Personalised Treatment with Artificial Intelligence project (OPERA, Horizon Europe, 2022-2027; https://www.opera.uio.no) aims to reduce the incidence and mortality of colorectal cancer through AI-enhanced screening. The MARC SE-Africa project (Horizon Europe, 2023-2027; https://www.marcse-africa.org) is designed to promote the translation of evidence of artemisinin and other drug resistance of public health significance to inform better malaria policy and practice before drug resistance increases the number of malaria cases and deaths in South East Africa and globally.
Bibliometric data according to the Google Scholar (reference source for UNIGE): A common group for the division of IM has been created, Division of General Internal Medicine (HUG-UNIGE) - Google Scholar, covering all three research groups. As members of this “EBM, Education & Health Services Research” can also collaborate in the two other research group of IM this common SMIG group allows a direct access to each individual investigator.
Current funding: Horizon Europe (EDCTP & HADEA), World Health Organization (WHO), Swiss National Science Foundation (SNSF), Canadian Institutes of Health Research (CIHR), HUG Private foundation, R&D hospital program, IM private funding, Norwegian Research Council, MAGIC Evidence Ecosystem Foundation.
Collaborations : Our group is intensely collaborating within the DMED and the institution, notably in co-supervision of MDs with Prof Delphine Courvoisier. Prof Agoritsas is also co-supervisor of PhD in nursing science of Mélanie Verdon. In Switzerland, we contribute to a growing Evidence Synthesis Network, including collaborations with Nicolas Rondodi, Georgia Salanti, Reto Auter (Bern); Mathias Briel, Lars Hemkens, Alain Amstutz (Basel); Kevin Selby, Marie-Anne Durand (Lausanne). Prof Agoritsas sits on the Swiss Cancer Screening Committee (https://cancerscreeningcommittee.ch). We have broad international collaboration through MAGIC, with staff collaborators spread out in Switzerland, Norway, UK, Portugal, Belgium, Canada, USA, Columbia. We provide methodological guidance for a portofolio of guideline across several WHO groups, and work with many other organisations such as ASCO, Danish Health Authority. We have coordinated contribution for >450 clinicians and researchers worlwide in panels of the BMJ Rapid Recommendations, and lead several key Work Packages in Horizon Europe projects, involging many European academic partners and leaders. Prof Agoritsas is part-time (upaid) faculty at McMaster University where co-supervises PhDs, and works directly with Prof Gordon Guyatt, co-founder or GRADE and member of the MAGIC board, and Prof Alfonso Iorio. Direct collaboration on Shared Decision making also involve Profs Glyn Elwyn and Victor Montori.
Selected relevant publications:
1. Agoritsas T, et al., Decision aids that really promote shared decision making: the pace quickens. BMJ. 2015 Feb 10;350:g7624.
2. Agoritsas T et al., Adjusted Analyses in Studies Addressing Therapy and Harm. Users’ Guides to the Medical Literature. JAMA. 2017;317(7):748-759.
3. Agoritsas T, et al., UpToDate adherence to GRADE criteria for strong recommendations: an analytical survey. BMJ Open. 2017 Nov 16;7(11):e018593.
4. Lamontagne F, Agoritsas T, Macdonald H, et al. A living WHO guideline on drugs for covid-19. BMJ. 2020 Sep 4;370:m3379.
5. Vandvik PO, Askie L, Glen F, Tendal B, Agoritsas T. COVID-19: living guidelines help fix cracks in evidence pipeline. Nature. 2021 Jul;595(7866):172.
6. Coen M, Sader J, Junod-Perron N, Audétat MC, Nendaz M. Clinical reasoning in dire times. Analysis of cognitive biases in clinical cases during the COVID-19 pandemic. Intern Emerg Med. 2022 Jun;17(4):979-988.
7. Owen A, Diaz JV, Guyatt G, Lamontagne F, Stegemann M, Vandvik PO, Agoritsas T. WHO Living Guidelines on antivirals for COVID-19 are evidence-based. Lancet. 2022 Dec 17;400(10369):2196-2198
8. Busse JW, Casassus R, Carrasco-Labra A, Durham J, Mock D, Zakrzewska JM, Palmer C, Samer CF, Coen M, Guevremont B, Hoppe T, Guyatt GH, Crandon HN, Yao L, Sadeghirad B, Vandvik PO, Siemieniuk RAC, Lytvyn L, Hunskaar BS, Agoritsas T. Management of chronic pain associated with temporomandibular disorders: a clinical practice guideline. BMJ. 2023 Dec 15;383:e076227.
Scientific and technical collaborators: Pauline Gosselin, PhD, research coordinator and Tamara Mann, research nurse. Both are involved with the 3 groups of the internal medicine research platform. Clément Buclin, MD-PhD candidate (Unige). Thomas Agoritsas is the Chair and Founder of the MAGIC Evidence Ecosystem Foundation (www.magicevidence.org), an international non-for profit organization, which conducts research in guideline methodology and implementation. MAGIC staff has 32 members in 5 countries (17 research, 15 technical, www.magicevidence.org/about/), contributing to the workforce and activities of this group.
