Insulin cells don’t need to team up
UNIGE scientists show that insulin-producing beta cells don’t need other pancreatic endocrine cells to stabilise blood sugar levels.
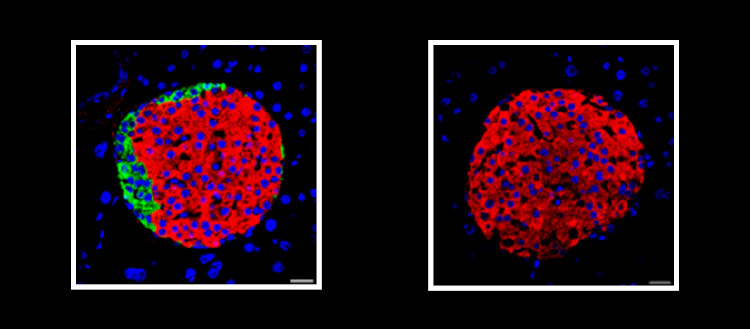
© Laboratoire Pedro Herrera – UNIGE. Pancreatic islets from adult mice. Top, islet composed of different types of endocrine cells. Bottom, islet composed solely of beta cells.
Our glycaemic balance is based on the ability of the pancreatic beta cells to detect glucose and secrete insulin to maintain our blood sugar levels. If these cells malfunction, the balance is broken, and diabetes develops. Until now, the scientific community agreed that beta cells needed the other hormone-producing cells of the pancreas to function properly. A team from the University of Geneva (UNIGE) has demonstrated the opposite: in adult mice whose pancreas contains only beta cells, glycaemia regulation and insulin sensitivity are even better than in standard animals. These results, which open major clinical prospects, can be read in the journal Nature Metabolism.
In 2010, the team led by Pedro Herrera, a professor in the Department of Genetic Medicine and Development and in the Diabetes Centre at the UNIGE Faculty of Medicine, discovered the remarkable ability of pancreatic cells to change function. If beta cells die prematurely, the endocrine cells normally responsible for producing other hormones, such as glucagon or somatostatin, can start producing insulin.
‘‘Until now, it was thought that the differentiated adult cells of an organism could not regenerate and reorientate themselves functionally. Pharmacologically triggering this cellular plasticity could therefore form the basis of an entirely new therapy for diabetes. But what happens if all the cells of the endocrine pancreas abandon their original function to start producing insulin? It is what we wanted to find out in our new study,’’ explains Pedro Herrera.
Non-beta cells are not essential
It was accepted that beta cells could only function correctly in the presence of the other hormone-producing cells – alpha, delta and gamma cells – grouped together in islets within the pancreas. ‘‘To verify this, we produced mice in which, when they reach adulthood, all the non-beta cells in the pancreas can be selectively eliminated to observe how the beta cells manage to regulate glycaemia,’’ explains Marta Perez Frances, a researcher in Pedro Herrera’s laboratory and first author of this work. ‘‘Surprisingly, not only were our mice perfectly capable of managing their blood sugar levels effectively, but they were even healthier than the control mice!”
Even when fed a high-fat diet or tested for resistance to insulin – one of the main markers of diabetes – these mice showed improved sensitivity to insulin in all the target tissues, and particularly in adipose tissue. Why? “There is an adaptation process in which the body recruits other hormonal cells from outside the pancreas to cope with the sudden reduction in glucagon and other pancreatic hormones,’’ notes Pedro Herrera. ‘‘But this clearly shows that non-beta cells of the pancreatic islets are not essential for maintaining glycaemic balance.’’ These results are surprising and challenge the prevailing conception up until now.
Emerging new therapies
Naturally, around 2% of pancreatic cells change their function in the event of insulin deficiency. The challenge is now to identify a molecule capable of inducing and amplifying this conversion. Another strategy would be to differentiate stem cells in vitro to produce new beta cells before transplanting them into the patients. ‘‘Our results are proof that strategies focusing on insulin cells could really pay off,’’ enthuses Pedro Herrera. ‘‘The next stage of our work will therefore involve establishing the molecular and epigenetic profile of non-beta cells from diabetic and non-diabetic individuals in the hope of identifying the elements which could make it possible to induce the conversion of these cells in the pathological context of diabetes.’’
3 Sept 2024