Structure-Based Design and Synthesis of Stapled 10Panx1 Analogues for Use in Cardiovascular Inflammatory Diseases
SUMMARY
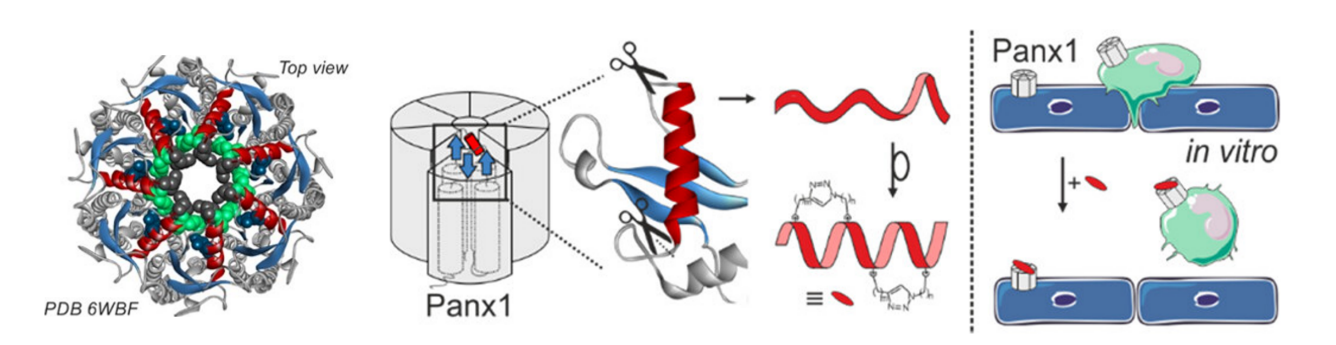
Pannexin1 (Panx1) is an important transmembrane protein mediating cellular communication through the formation of heptameric channels. Disproportionate opening of these channels has been associated with cardiovascular inflammatory pathologies in which extracellular ATP signaling plays a key role. Following a rational design, a series of macrocyclic (‘stapled’) peptidomimetics of 10Panx1, the most established peptide inhibitor of Panx1 channels, were synthesized to pinpoint where the side chain-to-side chain triazole-based crosslinks needed to be introduced for optimal Panx1 channel inhibition. Two macrocyclic analogs SBL-PX1-42 and SBL-PX1-44 outperformed the linear peptide inhibitor 10Panx1. During in vitro ATP release and Yo-Pro-1 uptake assays in a Panx1-expressing tumor cell line, both compounds revealed to be promising bidirectional inhibitors of Panx1 channel functions, able to induce a two-fold inhibition, as compared to the native 10Panx1 sequence. Moreover, both lead peptidomimetics revealed to be specific to Panx1 channels when tested on cardiomyocyte-like cells devoid of Panx1. The introduction of macrocyclization within the peptide backbones increased helical content and enhanced in vitro proteolytic stability in human plasma (>30-fold longer half-lives, compared to 10Panx1). Efforts to determine the optimal triazole-based compound have led to the synthesis of a ‘double stapled’ peptide, SBL-PX1-206. By measuring THP-1 monocyte adhesion to TNF-α-activated endothelial cells, the latter inhibited ATP release from endothelial cells thus efficiently reducing monocyte adhesion to an activated endothelial monolayer making it a promising candidate for future in vivo investigations in animal models of cardiovascular inflammatory disease.
This work was lead by Professor Brenda Kwak, Department of Pathology and Immunology and Geneva Center for Inflammation Research, UNIGE Faculty of Medicine, and Steven Ballet, Research Group of Organic Chemistry, Departments of Chemistry and Bioengineering Sciences, Vrije Universiteit Brussel. The research was funded by Research Council of the VUB through the Strategic Research Program, the European Union’s Horizon 2020 Future and Emerging Technologies Programme PANACHE.
Full article: https://pubs.acs.org/doi/10.1021/acs.jmedchem.3c01116
Why is this important?
Cardiovascular disease remains the leading cause of mortality and morbidity worldwide, and inflammation is an important driving force in multiple cardiovascular disorders. ATP is a central signalling molecule that orchestrates the initiation and resolution of the inflammatory response by enhancing activation of the (NLRP3) inflammasome and leukocyte recruitment. ATP can be released from cells in a controlled manner through pannexin1 (Panx1) channels. Hence, these channels may represent a novel anti-inflammatory target. Unfortunately, the vast majority of Panx1 channel blocking compounds lack specificity and/or serum stability, which limits their application. This study reports the first peptidomimetic with high plasma stability and target specificity. The potential of this peptidomimetic as a future means for selective Panx1 inhibition for therapeutic purposes will be further explored by Brenda Kwak’s research group in the context of the H2020 Future and Emerging Technologies Programme PANACHE project (http://www.panache-project.eu/).
21 Sept 2023