Negative staining TEM
Principles:
Small volume of the sample suspension is absorbed on glow-discharged EM grid, washed several times on drops of ultrapure water and incubated on the drop(s) of negative staining solution containing heavy metal salt (uranyl acetate). Finally, the staining solution is completely blotted off and air-dried.
This leaves thin layer of heavy metal stain surrounding the sample and resulting to the "white" appearance of the sample against darker backround (as opposite of heavy metal stained membranes on the ultra-thin sections) (Fig. 1).
Application:
First choice method to study the morphology and structure of:
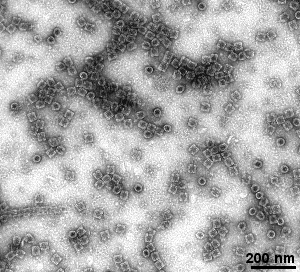
- purified subcellular components (e.g. ribosomes, mitochondria, ...)
- isolated macromolecules and protein complexes (e.g. cytoskeletal components, large intracellular complexes, ...)
- in vitro reconstituted complexes or assemblies
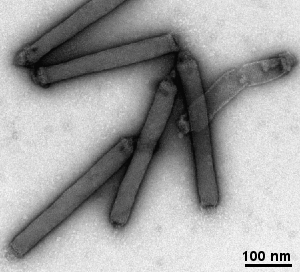
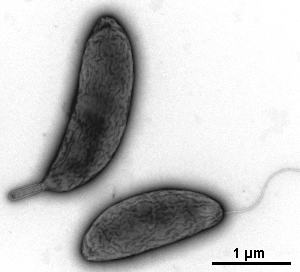
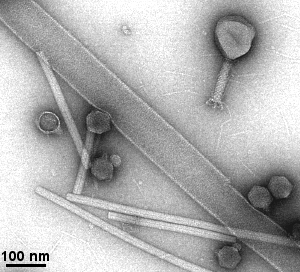
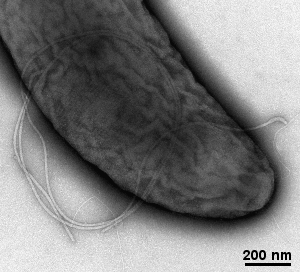
- viruses, bactreriophages, bacteria's pili and flagellum, ...
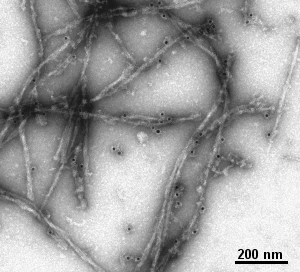
- filaments (e.g. actin, microtubules, neurofilaments, ...
Method:
- EM grid coated with both, plastic support film and carbon film is glow-discharged for 1 min. This treatment render surface of the grid negatively charged and hydrophilic which facilitates the absorption of the sample on the grid.
- Small amount of sample suspension (5 µL) is applied on freshly glow-discharged surface of the grid and incubate for 1 min. Excess of the liquid is removed by brief blotting with filter paper (Fig. 1A).
- Grid is immediatelly washed 3 times on drops of ultrapure water (to remove any traces of unabsorbed sample and salts or additives) with brief blotting in between each wash (Fig. 1B left).
- Grid is incubated on 1st drop of the heavy metal salt solution (20-40 µL) for 15 sec, briefly blotted and then on 2nd drop of the heavy metal salt solution (20-40 µL) for additional 45 sec (1 min in total) (Fig. 1B right).
- Finally, excess of the heavy metal salt staining solution is blotted with filter paper until no more wetting of the filter paper is detected and grid is fully air-dried. This will leave thin layer of the heavy metal salt around the sample (Fig. 1C).
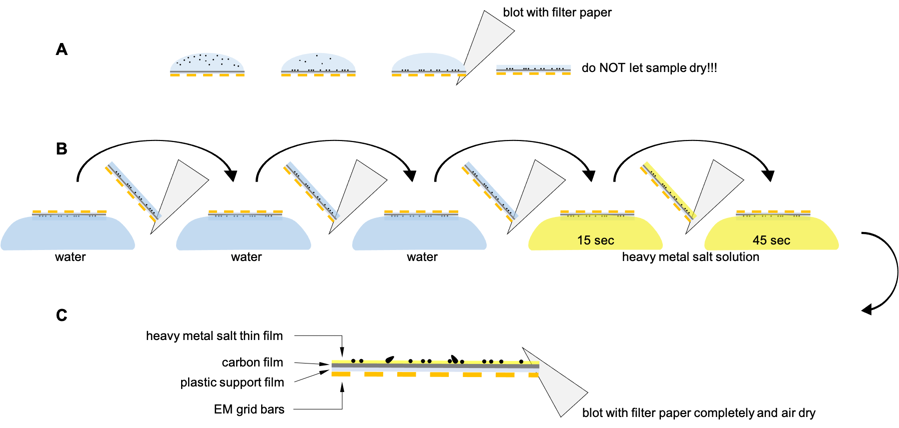 |
| Fig. 1: Principle of the negative staining |
Notes:
- Sample:
- concentration: >0.2 mg/mL (always easier to dilute the sample)
- low salt <150 mM
- ideally to avoid detergents
- Most commonly used negative staining solution is uranyl acetate (1-2 % aqueous solution, acidic pH 4)
- Other stains with adjustable pH (but always made fresh just before use)
- phosphotungstic acid
- uranyl formate
- ammonium molybdate
- Possible to combine with immuno labelling with seconary antibodies coupled with colloidal gold before heavy metal staining
- To image small proteins <50 kDa can be difficult
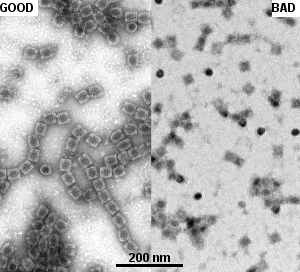
- Do not let grid completely dry before incubation with staining solution:
- this will damage the sample
- lead to produce positive contrast and quite blurry appearance, lost of details (see Fig. 2)
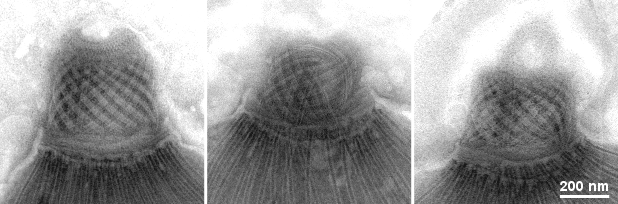
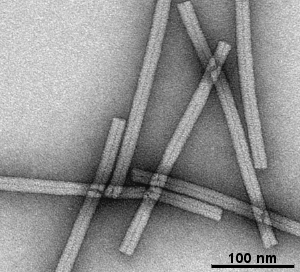
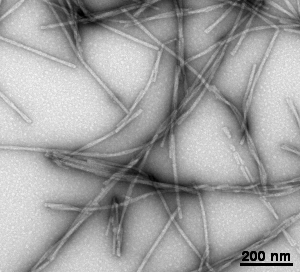
Gallery of different samples prepared by negative staining