Scanning Electron Microscopy Imaging
Principles:
Primary electron beam is scanning the sample pixel by pixel, line by line and secondary electrons bounced off the sample surface are collected via secondary electrons detector to produce the topology image of the sample.
Application:
Method of choice to study the surface morphology and structure of:
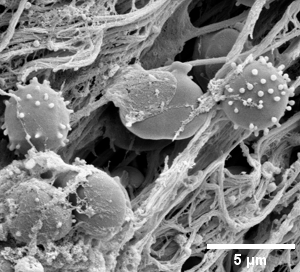
- cells and tissue
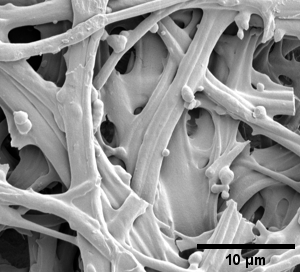
- filaments (e.g. fibrinogen filament of the blood clot, purified, from patients ...)
- morphology of aorta ...
- bacterial biofilms
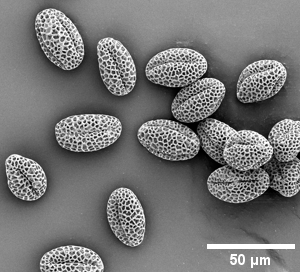
- pollen
- insect
- organoids
- ...
Method:
- Small piece (1 - 2 mm) of tissue or cell pellet or attached cells on coverslip are chemically fixed with 2.5% glutaraldehyde and 2% paraformaldehyde mixture in 0.1 M Na-cacodylate buffer for at least 1 h at room temperature (or overnight at 4 °C).
- Postfixation with 1% tannic acid in 0.1 M Na-cacodylate buffer for 1 h.
- Postfixation with 1% Os04 in cacodylate buffer for 1h at room temperature
- Dehydration with ethanol grade series: 50%, 70%, 80%, 90% for 5 min each and 100% 3 times for 10 min each.
- Then samples in absolute ethanol are transferred into critical-point drying (CPD) machine to completely dry the sample replacing the liquid ethanol with CO2 without forming liquid-gas interface and thus preventing to collaps the 3D organization of the sample.
- After CPD drying, samples need to be immediately mounted on SEM aluminum stabs (double-sided carbon tape or carbon or silver conductive paint).
- Finally, sample's surface have to be metal coated (typically ~ 3 nm layer of gold) to by conductive in SEM chamber and to prevent building the charge during the imaging with Helios 660 Nanolab DualBeam FIBSEM instrument.
Notes:
- glass coverslips can break inside the pressurized chamber of the CPD machine, better use the plastic coverslips
- After CPD treatment, samples are very light and easily electrostatic charged (can easily "fly away")
- Once sample is critical-point dried or mounted on the SEM stub, always HAVE TO BE KEPT in dessicatotor until imaging.
Gallery of different samples prepared for SEM
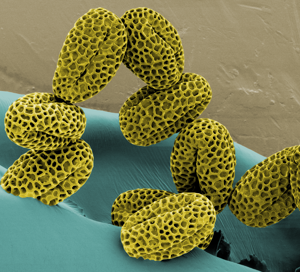
Lillac pollen artificial colours |
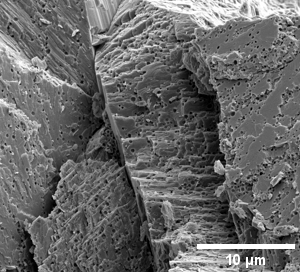
Chicken egg shell: outer surface |
|