Subcellular Control of Receptor Signaling
G protein-coupled receptors (GPCRs), also called seven-transmembrane receptors, comprise nature’s largest receptor family and are activated by a remarkable diversity of ligands that include neurotransmitters, hormones, odorants, ions, and photons. GPCRs play essential roles in physiology and disease and represent the most common class of therapeutic targets.
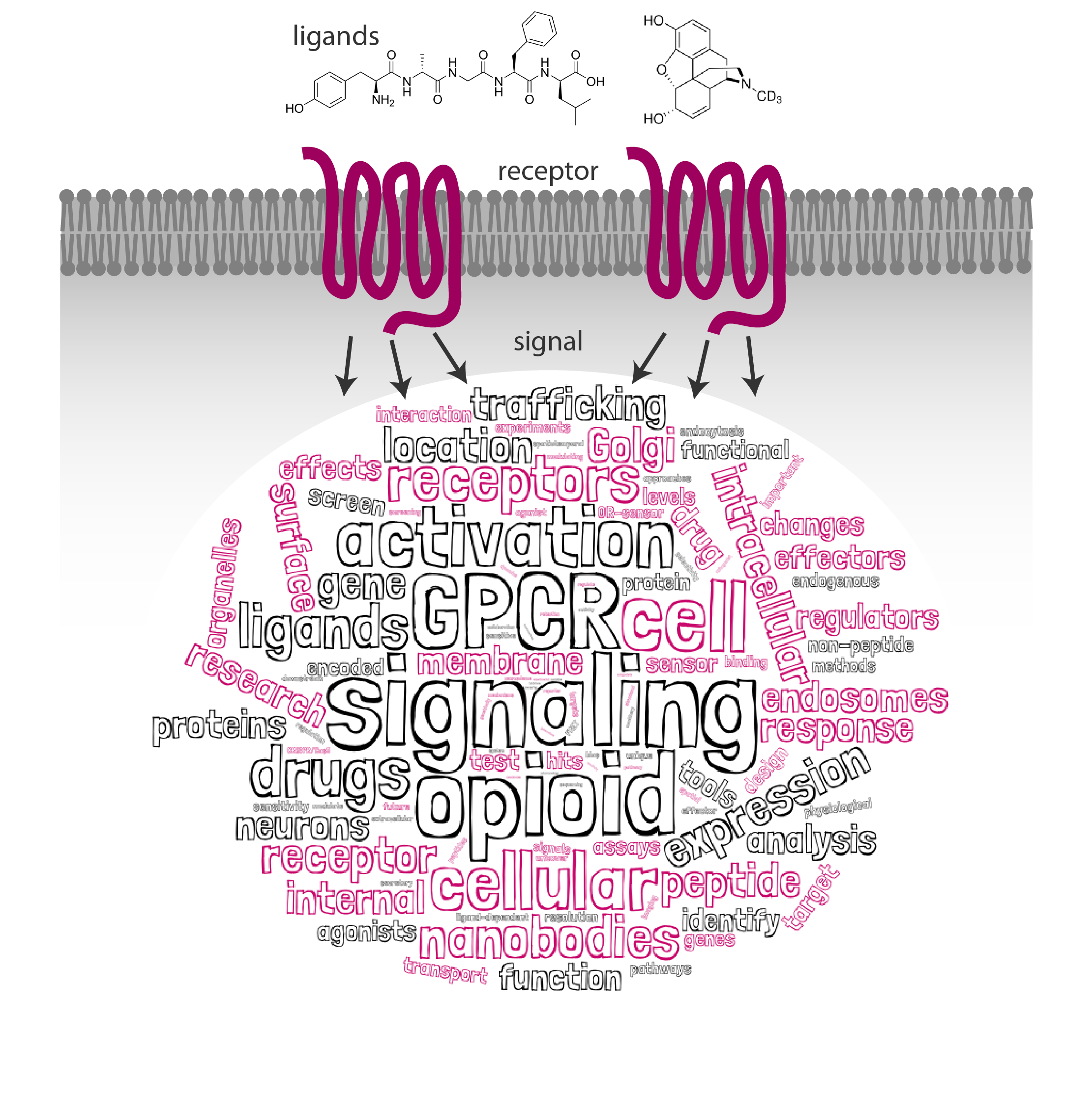
An emerging principle in GPCR biology is that chemically distinct ligands that activate the same cognate receptor can produce different signaling responses. Ligand-selective signaling is motivating ongoing drug development campaigns, however, it remains largely unknown how exactly distinct ligands drive discrete signaling pathways in the cellular context.
In our laboratory, we are studying how the precise subcellular location of receptor signaling defines ligand-selective effects. In particular, we focus on the physiologically and pharmacologically important GPCR family called opioid receptors. Opioid receptors are the target of opioid drugs (e.g. morphine or fentanyl) that are the most effective agents known for alleviating pain but produce significant toxicity and have high abuse potential. Previously we have shown that opioid drugs differ from endogenous peptides in the cellular location at which they drive receptor activation. It suggests that some drug-specific effects and abuse responses may be explained by what cellular receptor pool a certain drug is interacting with.
Now we aim to unravel how location-specific signaling contributes to opioid drug action and pathology. In this newly established SNSF Eccellenza-funded group, we will combine cutting edge live-cell microscopy, novel nanobody-based biosensors, pharmacology, neurobiology, and genomic approaches to dissect clinically important GPCR signaling systems in the cellular context.