Internal clocks drive beta cell regeneration
Scientists from UNIGE and HUG identify the essential role of circadian clocks in the regeneration of insulin-producing cells.
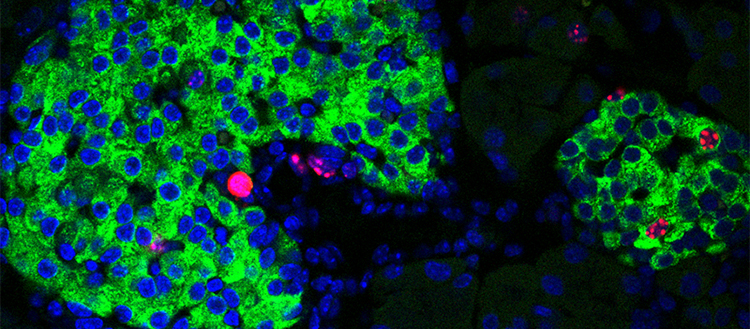
Image of pancreatic islets showing proliferation markers (in red staining) in the nuclei (in blue) of insulin-producing-cells (in green). © UNIGE/Dibner
Certain parts of our body, such as the skin or liver, can repair themselves after a damage. Known as cell regeneration, this phenomenon describes how cells that are still functional start to proliferate to compensate for the loss. For the past 30 years, scientists have been investigating the regenerative potential of beta cells, pancreatic cells in charge of the production of insulin. Beta-cell population is indeed partially destroyed when diabetes occurs, and regenerating these cells represents an outstanding clinical challenge. By studying diabetic mice, scientists from the University of Geneva (UNIGE) and the University Hospitals of Geneva (HUG), observed that this regeneration mechanism was under the influence of circadian rhythms - the molecular clocks regulating metabolic functions according to a 24-hour cycle of alternating day-night. In addition, the scientists identified the essential role of the core clock component BMAL1 in this process. These results, to be read in the journal Gene and Development, allow new perspectives to be envisaged to promote beta cell regeneration.
Compensatory proliferation, in which cells begin to actively divide to replace those that have been damaged, is a biological mechanism that is both well-known and poorly understood. «And this is particularly true for pancreatic beta cells, whose regenerative mechanism stays largely unexplored despite decades of research,» explains Dr Charna Dibner, head of the Circadian Endocrinology Laboratory at UNIGE Faculty of Medicine’s the Departments of Medicine and Cell Physiology and Metabolism, as well as at the Diabetes Centre, and at the HUG. «However, deciphering this phenomenon and above all finding out how to promote it could be a game changer for controlling diabetes.»
Day-night rotation is essential
To explore the connection between internal biological clocks and beta cell regeneration, Charna Dibner’s team first observed two groups of mice with only 20% beta cells remaining after targeted massive ablation. Mice in a first group were arrhythmic, whereas the control group had perfectly functional clocks. «The result was very clear: the mice bearing dysfunctional clocks were unable to regenerate their beta cells, and suffered from severe diabetes, while the control group animals had their beta cells regenerated; in just a few weeks, their diabetes was under control,» says Volodymyr Petrenko, a researcher in Dr. Dibner’s laboratory and the leading scientist in this study. By measuring the number of dividing beta cells across 24 hours, the scientists also noted that regeneration is significantly greater at night, when the mice are active.
The BMAL1 gene, metronome of cell activity
The arrhythmic mice were lacking the BMAL1 gene, which codes for the protein of the same name, a transcription factor known for its key action in the functioning of circadian clock. «Our analyses show that the BMAL1 gene is essential for the regeneration of beta cells,» adds Volodymyr Petrenko. In addition, large-scale transcriptomic analyses over a 24-hour period, conducted in collaboration with Prof. Bart Vandereycken at the Mathematics Department of the UNIGE, revealed that the genes responsible for regulating cell cycle and proliferation were not only upregulated, but also acquired circadian rhythmicity. “BMAL1 seems to be indeed central for our investigation,” stresses Charna Dibner. “However, whether the regeneration requires functional circadian clocks themselves, or only BMAL1, whose range of functions goes beyond clocks remains unclear. That is what we would like to find out at present.” The scientists also want to explore the function of alpha cells, which produce glucagon, the hormone that antagonises insulin, in this model. The arrhythmic mice indeed showed very high levels of glucagon in the blood. “A detailed understanding of these mechanisms must now be pursued, in an attempt to explore the possibility of triggering beta cell regeneration in humans in the future” conclude the authors.
11 Nov 2020