Cystic fibrosis: why so many respiratory complications?
By demonstrating the key role of Vav3 protein, UNIGE researchers highlight how the protein Vav3 creates bacterial docking stations to facilitate lung infections in cystic fibrosis.
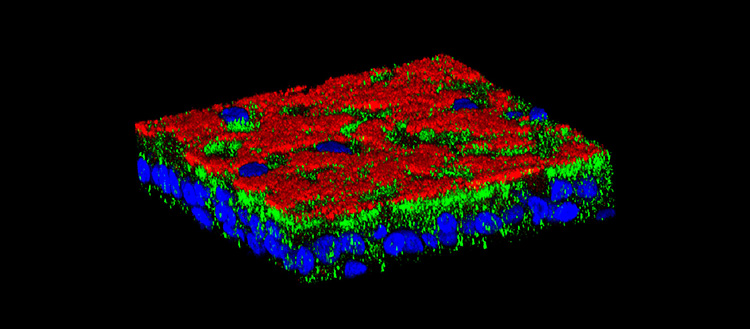
The protein Vav3 (in green) creates «bacterial docking stations» on the surface (in red) of respiratory cells (nuclei in blue) that facilitate airways’ infection in patients with cystic fibrosis. © UNIGE, laboratoire Chanson
Cystic fibrosis, one of the most common genetic diseases in Switzerland, causes severe respiratory and digestive disorders. Despite considerable therapeutic advances, this disease still reduces life expectancy, in particular due to life-threatening respiratory infections. Scientists from the University of Geneva (UNIGE) have discovered the reason for this large number of lung infections: a protein, Vav3, promotes these infections by creating a “bacterial docking station” on airways’ surface. Inhibiting this protein might prevent bacteria from docking on airways’ surface and causing recurrent infections. These results, to be read in the journal Cell Reports, pave the way of interesting therapeutic prospects for limiting respiratory complications in people with cystic fibrosis.
Cystic fibrosis, affecting more than 700,000 people worldwide, is one of the most common genetic diseases in Switzerland. It stems from mutations in the gene responsible for a protein that participates in the secretion of mucus, making it abnormally thick. The alteration of this single gene leads to severe respiratory and digestive problems and limits both life quality and life expectancy of those affected. In lungs for example, hyperviscous mucus stagnates and obstructs airways.
Recurrent infections
Mucus accumulation does not only obstruct airways, it also promotes persistent lung infections. Despite major therapeutic advances, these lung infections remain frequent and serious. They are mostly due to a bacterium known for its resistance to antibiotics, Pseudomonas aeruginosa. “While it is known that mucus viscosity plays a role in trapping bacteria, the reason why they anchor so easily to airways was unknown,” explains Marc Chanson, Professor at the Department of Cell Physiology and Metabolism of the Faculty of Medicine of the UNIGE. “Anchoring of Pseudomonas aeruginosa to airways’ cells is the starting point for these often fatal infections. Understanding this process could help preventing their occurrence.”
A bacterial docking station
UNIGE researchers compared airway cells from sick people with healthy cells. “The whole project began when we found that the protein Vav3, which had not been shown to be involved in this disease until now, was over-expressed in sick cells,” enthuses Mehdi Badaoui, researcher in Prof. Marc Chanson’s team and first author of this work. After numerous in vitro analyses, scientists discovered the key role of this protein: it directs the construction of a true bacterial docking station. In concrete terms, Vav3 forces two other proteins, fibronectin and integrin b1, to associate with it on cell surface and create a complex that promotes Pseudomonas aeruginosa infections. “This is the first time that a mechanism creating a favourable microenvironment for a bacterium before it even arrives has been observed,” says Marc Chanson. “This might explain the high number of chronic lung infections in people with cystic fibrosis.”
Inhibiting Vav3 to limit respiratory infections
How to build on this mechanism to develop therapeutic options? By inhibiting Vav3 in sick cells, scientists succeeded in preventing the expression of the two other proteins that make up the docking station. “And, indeed, the absence of this structure limits the adhesion of Pseudomonas aeruginosa”, adds Mehdi Badaoui. Although the exact link between the protein Vav3 and the genetic defect that causes cystic fibrosis has yet to be determined, this discovery is a promising therapeutic target for limiting respiratory complications.
This work has been made possible, in particular, thanks to the support of the Swiss National Science Foundation (SNSF) and the Swiss Society for Cystic Fibrosis.
13 Jul 2020
