Complex genetic programmes at the root of our movements
A UNIGE team has discovered the genetic programmes that allow motor neurons to retract from the spinal cord. This discovery opens up prospects for combating neurodegeneration.
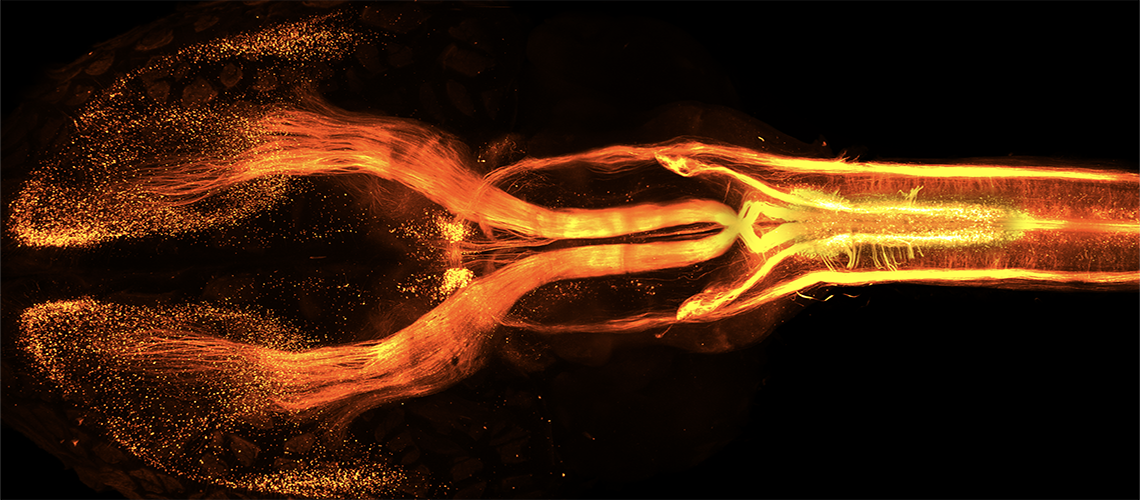
The motor cortex is made up of neurons responsible for muscle contraction. These neurons have cellular extensions called axons, which project from the cortex into the spinal cord. During brain development, some of these neurons retract their axons to project, not into the spinal cord, but into the brain. How does this happen? Neuroscientists at the University of Geneva (UNIGE) have discovered that everything is linked to genetic programming. Indeed, our genes define which parts of the cortex are dedicated to motor functions and which are not, by directing neuronal projections. This fundamental discovery, published in the journal Nature, opens up new avenues for countering motor disorders.
The cerebral cortex is the outer part of the brain responsible for higher cognitive functions, such as thinking, perception, decision-making, language and memory. It also processes sensory information and controls movement. To do this, it dedicates part of its volume to movement: the motor cortex. This is where the neurons responsible for muscle contraction — the corticospinal neurons — project to the spinal cord. Despite the compartmentalisation of the cortex, corticospinal neurons are found outside the motor cortex. Why is this?
Selection during development
To answer this question, the neuroscientists focused on the mouse. ‘‘The technologies currently available do not allow to address these questions in humans. Corticospinal neurons are highly conserved across species and can therefore be studied in rodents,” says Denis Jabaudon, full professor in the Department of Basic Neuroscience at the UNIGE Faculty of Medicine and initiator of the study.
Using approaches that make brain tissue transparent and allow specific staining of neuron subtypes, the research team first studied the evolution of corticospinal projections during brain development. “We have thus confirmed a fascinating observation made several decades ago, but little known to neuroscientists,” explains Denis Jabaudon. At the start of brain development, neurons in the cortex project into the spinal cord. Those that will form the future motor cortex remain there, while those that will make up the rest of the cortex gradually retract. In the end, in an adult brain, some corticospinal neurons can act as far away as the spinal cord, and others with a shallower range of action, which remain in the brain itself.
A dedicated genetic programme
Denis Jabaudon’s team then compared the genes expressed by these two types of neurons and identified a family of genes responsible for their ability to retract. “Without them, these neurons would remain anchored in the spinal cord during development, and our cortex would probably be deprived of its higher cognitive functions,” adds Denis Jabaudon.
To demonstrate the importance of this genetic programme, the researchers focused on three genes and, using gene-editing techniques – CRISPR-Cas9 – were able to modulate their expression in neurons with projections into the spinal cord. “This was a major technical challenge and represents a new way of assessing the influence of a set of genes on cellular mechanisms,” says Denis Jabaudon. It was thus possible to force the retraction of neurons from the spinal cord into the brain.
An essential discovery
‘‘Understanding how corticospinal neurons emerge during development and how they project into our central nervous system is important, as they are essential for fine motor skills. However, they are highly sensitive to spinal cord injury or to the consequences of amyotrophic lateral sclerosis, a disease that causes a progressive paralysis of muscles,” says Denis Jabaudon. ‘‘In this study, we succeeded in forcing the neurons to retract. But everything suggests that the opposite could also be done, which opens up fascinating possibilities,’’ he adds. The research team is now considering reprogramming neural cells in other contexts, such as neurodegenerative diseases.
