Research
Ultrafast two-dimensional infrared spectroscopy (2D-IR) is our group's main workhorse. This technique allows us to resolve vibrational couplings, orientation, population exchange and energy transfer, and solvation dynamics (through the observed lineshapes in the spectra).
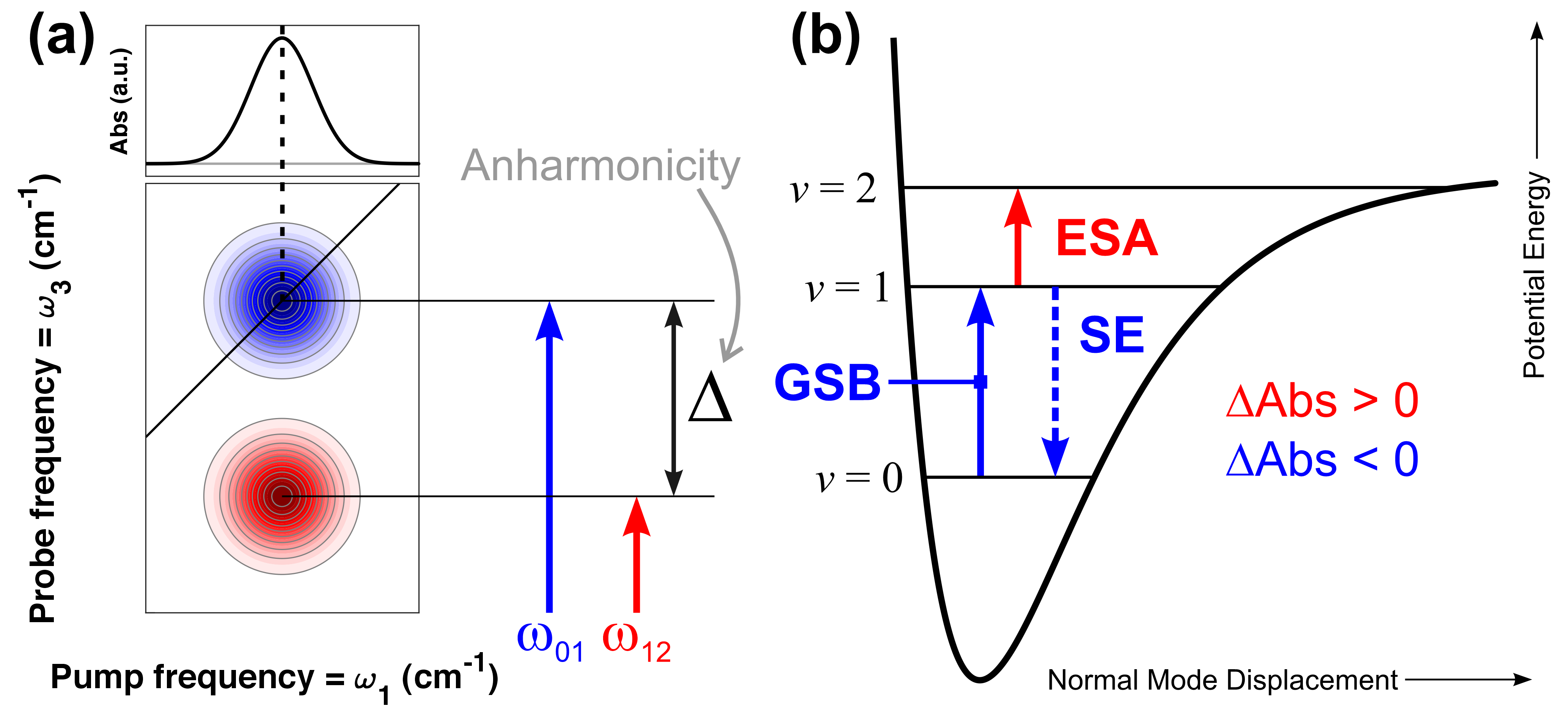
In a 2D-IR spectrum (a), every diagonal peak contains two contributions: a negative transient absorption signal due to the GSB/SE of the 0-1 transition; and a positive, anharmonically shifted ESA, due to the 1-2 transition. In conventional potential energy surfaces (b), the anharmonicity is positive, and thus the ESA is shifted to lower frequencies (wavenumbers). Cross peaks indicate vibrational couplings and/or population transfer, depending on their kinetics (analogous to COSY and NOESY cross peaks in 2D-NMR).
Transient 2D-IR (tr2DIR) is an extension of 2D-IR spectroscopy, where we now record a 2D-IR spectrum of the excited-state species or photoproducts that are generated with an actinic UV/Vis pulse.
Thanks to pulse shaping, we can record 2DIR/tr2DIR spectra in both time and frequency domains. Each approach has its own (dis)advantages. Furthermore, we can measure in the rotating frame and use t1 undersampling to greatly increase acquisition speed. [Read more in the Tutorials section]
2D Vibrational-Electronic spectroscopy (2D-VE) represents a mix between the study of molecular vibrations and their involvement in electronic excitations, allowing us to resolve vibronic couplings---central to understanding photochemical reactivity under certain circumstances.
2D-VE was originally developed by the Khalil group (U. of Washington).
(Under construction)
The coupled transfer of an electron and a proton during a (photo)chemical reaction is a way nature found to balance charges and avoid large solvation penalties. In our group, we focus on understanding ground- and excited-state PCET reactions (as well as their constituent electron and proton transfer reaction steps).
We combine time-resolved multidimensional spectroscopy and synthetic modifications on the molecules of interest to understand the role of structural factors, as well as the role of electronic, vibrational and vibronic couplings in steering excited state reactivity.
We believe that being able to control these properties (with e.g. another excitation pulse) will allow us to alter the reactivity of the molecule without changing its identity, and provide a rich array of mechanistic insight.
Proton and electron transfer reactions are well-known phenomena. Most of the studies, however, have been carried out in "conventional" organic solvents or water. In our group, we are interested in understanding how proton and electron transfer reactions behave in non-conventional solvents, such as deep eutectic liquids (DEL) and ionic liquids (IL).
These solvents offer unique properties, including their tunability (DELs and ILs can be designed to have specific properties like acidity or polarity, influencing how they interact with solute molecules), stability, and a more intricate temperature response.
By understanding how ET and PT reactions are affected by the nature of these solvents, we can gain valuable mechanistic insights, leading us to design new reactions and catalysts for various applications, like organic synthesis and energy conversion.
After electronic excitation, organic dyes typically relax following a set of "standard" steps, which have been extensively studied in the past. The role of higher multiplicity states (i.e triplets, quintets, etc.) is often negligible for organic molecules with light atoms.
The fate of electronically excited states in coordination compounds, however, is complicated by the presence of the metal atoms, which promote fast intersystem crossing to manifolds of higher multiplicity.
In our group, we use ultrafast vibrational and electronic spectroscopies to understand the deactivation pathways of coordination compounds and their structure-property relationships. Armed with this knowledge, we can design, synthesise and characterise complexes that can find vast applications, ranging from their use as photosensitisers in photocatalytic systems, to luminophores in OLEDs.
Apart from investigating our own molecules, we also collaborate with other synthetic groups to understand the excited-state dynamics of novel photoactive transition metal complexes. Feel free to contact us if you are interested in learning more about your molecules!
Transition Metal Hydrides are ubiquitous intermediates in many chemical, photochemical and electrochemical processes, playing a key role in water/proton reduction catalysis and CO2 reduction.
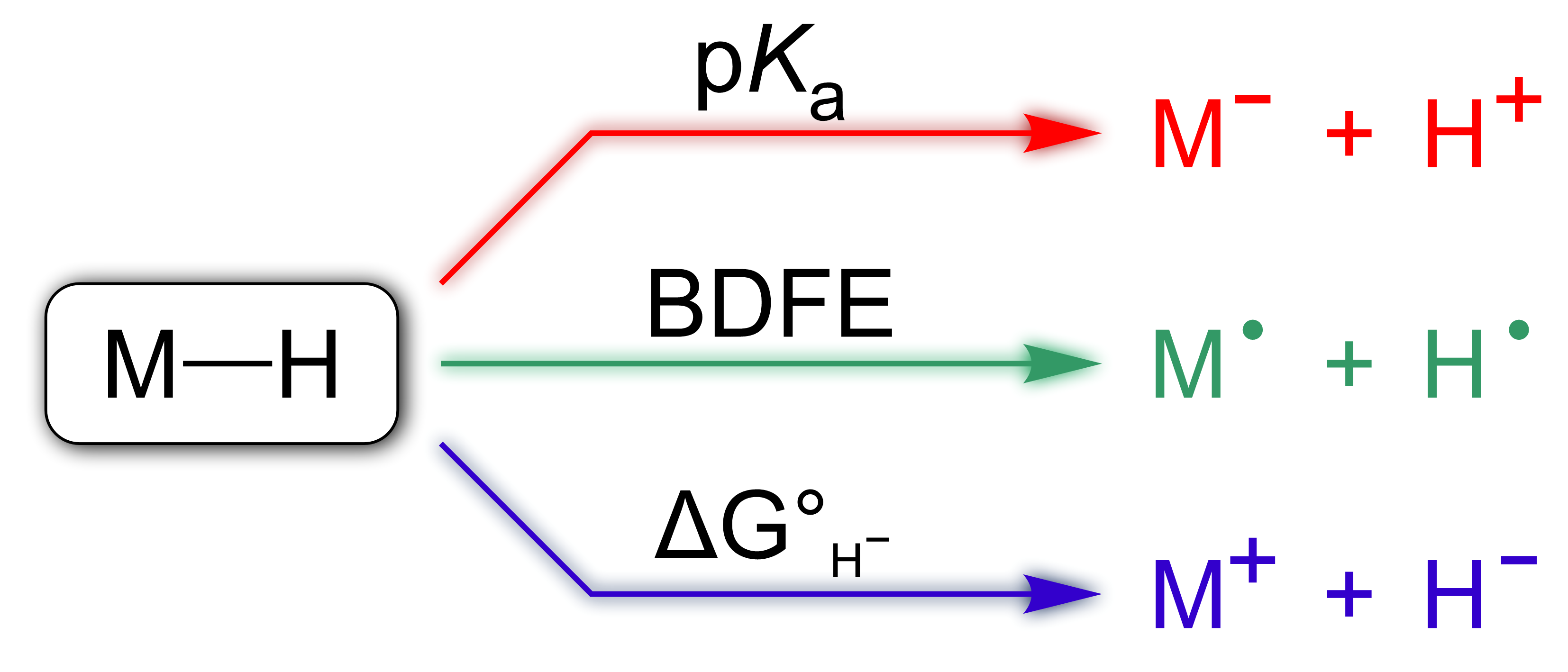 Metal hydrides can be formed (or can react) by three main pathways, controlled by the pKa, bond dissociation free energy (BDFE), and hydricity, respectively (see figure). The relative magnitudes of these three parameters depend strongly on the ability of the solvent to accommodate changes in the charge and/or electron density in the metal complex, as well as its capacity to solvate H+/·/-.
Metal hydrides can be formed (or can react) by three main pathways, controlled by the pKa, bond dissociation free energy (BDFE), and hydricity, respectively (see figure). The relative magnitudes of these three parameters depend strongly on the ability of the solvent to accommodate changes in the charge and/or electron density in the metal complex, as well as its capacity to solvate H+/·/-.
The M-H moiety has other interesting properties: its ambiguous polarisation, its ability to form hydrogen bonds, and—more importantly for us—the ultrafast dynamics of the M-H stretching vibrational mode(s). These modes directly report (thanks to 2D-IR spectroscopy) on the local field fluctuations around the M-H moiety, and can be used to determine the oxidation and protonation state of a metal hydride that can engage in PCET and/or PT/ET. In our group, we take advantage of these properties to monitor such reactions in real time.
The ultrafast dynamics of a molecule can be surprisingly dependent on its specific redox state.
This has been observed in studies of e.g. flavins. This research has shown that the flexibility of the flavin molecule's key ring structure plays a crucial role. In its oxidized state, the molecule undergoes ultrafast internal electron transfer within a few ps, followed by recombination. In contrast, in its reduced states, the excited state dynamics become slower---reaching the ns regime---and are influenced by the surrounding environment. This dependence of ultrafast dynamics on the redox state highlights the intricate interplay between a molecule's electronic structure and its behavior on ultrafast timescales.
In our group we aim to understand, using ultrafast multidimensional methods, the dependence of solvent--solute interactions on the redox state of the solute, as well as the role of these interactions in steering the photochemical reactions that can take place. In this way, we can design and synthesise more efficient catalysts, or fine-tune a photochemical reaction to obtain the desired outcome.
(Under construction)
