Publication 122
- Enantioselective iminium-catalyzed epoxidation of hindered trisubstituted allylic alcohols
Roman Novikov, Jérôme Lacour
Tetrahedron: Asymmetry 2010, 21, 1611-1618
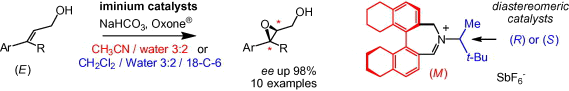
The reactivity of diastereomeric biaryl iminium cations made of a (Ra)-5,5′,6,6′,7,7′,8,8′-octahydrobinaphthyl core and exocyclic appendages derived from (S)- or (R)-3,3-dimethylbutyl-2-amine was investigated with hindered trisubstituted allylic alcohols—a class of alkenes which had not been previously studied in detail in epoxidation reactions with cyclic iminium catalysts (ee up to 98%). Surprisingly, generally strong matched/mismatched effects are observed not only in terms of reactivity but also on the enantioselectivity of the reaction (Δee up to 16%). Also, for the most hindered substrates, two sets of reaction conditions were tested in a preliminary study and little advantage was found in running reactions in MeCN/water instead of CH2Cl2/water/18-C-6. In any case, the presence of the hydroxyl group did not reveal any anchimeric effect.
DOI : 10.1016/j.tetasy.2010.06.002
archive ouverte unige:11037
