Publication 125
- (Cyclopentadienyl)ruthenium-Catalyzed Regio- and Enantioselective Decarboxylative Allylic Etherification of Allyl Aryl and Alkyl Carbonates
Martina Austeri, David Linder, Jérôme Lacour
Adv. Synth. Catal. 2010, 352, 3339-3347
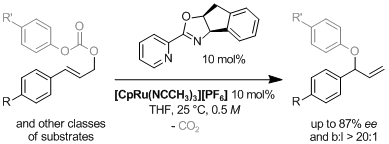
(Cyclopentadienyl)tris(acetonitrile)ruthenium hexafluorophosphate {[CpRu(NCMe)3][PF6]} or (cyclopentadienyl)(η6-naphthalene)ruthenium hexafluorophosphate {[CpRu(η6-naphthalene)][PF6]} in combination with a pyridine oxazoline ligand efficiently catalyze the decarboxylative allylic rearrangement of allyl aryl carbonates. Good levels of regio- and enantioselectivity are obtained. Starting from enantioenriched secondary carbonates, the reaction is stereospecific and the corresponding allylic ethers are obtained with net retention of configuration. An intermolecular version of this transformation was also developed using allyl alkyl carbonates as substrates. Conditions were found to obtain the corresponding products with similar selectivity as in the intramolecular process. Through the use of a hemi-labile hexacoordinated phosphate counterion, a zwitterionic air- and moisture-stable chiral ruthenium complex was synthesized and used in the enantioselective etherification reactions. This highly lipophilic metal complex can be recovered and efficiently reused in subsequent catalysis runs.
DOI : 10.1002/adsc.201000696
archive ouverte unige:12923
