Liste
Précédente Suivante
Publication 157
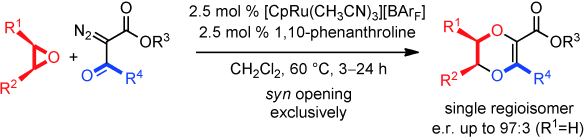
- [CpRu]-Catalyzed Carbene Insertions into Epoxides: 1,4-Dioxene Synthesis through SN1-Like Chemistry with Retention of Configuration
Thierry Achard, Cecilia Tortoreto, Amalia I. Poblador-Bahamonde, Laure Guénée, Thomas Bürgi, Jérôme Lacour
Angew. Chem. Int. Ed. 2014, 53, 6140-6144
[ This article was highlighted as a SynStory in Synform, 2014/10 (DOI: 10.1055/s-0034-1379040) ]

Rather than lead to the usual deoxygenation pathway, metal carbenes derived from α-diazo-β-ketoesters undergo three-atom insertions into epoxides using a combination of 1,10-phenanthroline and [CpRu(CH3CN)3][BArF] as the catalyst. Original 1,4-dioxene motifs are obtained as single regio- and stereoisomers. A perfect syn stereochemistry (retention, e.r. up to 97:3) is observed for the ring opening, which behaves as an SN1-like transformation.
DOI : 10.1002/anie.201402994
archive ouverte unige:37229
