Publication 161
- Modular synthesis of pH-sensitive fluorescent diaza[4]helicenes
Antoine Wallabregue, Petr Sherin, Joyram Guin, Céline Besnard, Eric Vauthey, Jérôme Lacour
Eur. J. Org. Chem. 2014, 6431-6438
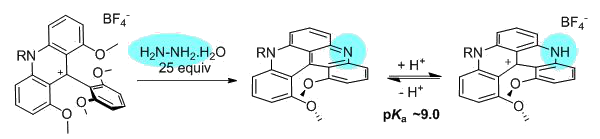
Using an particularly-facile N-N bond cleavage reaction, configurationally stable diaza [4]helicenes are prepared in two steps. The synthetic procedure, that uses hydrazine NH2NH2, is general, modular and highly tolerant to functional groups. The resulting helical quinacridines are dyes that present absorption and emission properties that can be modulated as a function of pH; the pink quinacridine and green protonated forms (pKa ~9.0) displaying distinct optical features in the near-IR region. Single enantiomers were obtained by a chiral stationary phase HPLC resolution. Absolute configuration assignment was realized by ECD spectra and a rather high racemization barrier was measured (ΔG‡ 30.7 ± 4.0 kcal.mol-1 at 140 °C).
DOI : 10.1002/ejoc.201402863
archive ouverte unige:40860
