Liste
Précédente Suivante
Publication 164
- Remote stereoselective deconjugation of α,β-unsaturated esters by simple amidation reactions
Mahesh Vishe, Radim Hrdina, Amalia I. Poblador-Bahamonde, Céline Besnard, Laure Guénée, Thomas Bürgi, Jérôme Lacour
Chem. Sci. 2015, 6, 4923-4928
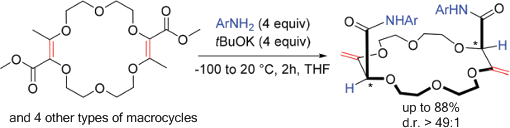
The thermodynamically disfavored isomerization of α,β-unsaturated esters to deconjugated β,γ-unsaturated analogues occurs readily when coupled to an amidation. Within the framework of macrocyclic derivatives, it is shown that 15, 16, and 18 membered macrocycles react with tBuOK and anilines to generate in one-pot the β,γ-unsaturated amides (yields up to 88%). Importantly, single (chiral) diastereomers are isolated (d.r. > 49:1, 1H NMR) irrespective of the size and nature of the rings showing an effective transmission of remote stereochemistry during the isomerization process. CSP-chromatographic resolution and absolute configuration determination by VCD are achieved
DOI : 10.1039/C5SC01118C
archive ouverte unige:74171
