Publication 183
- Stereoselective and Enantiospecific Mono- and Bis-C−H azidation of Tröger Bases. Insight on Bridgehead Iminium Intermediates and Application to Anion-Binding Catalysis
Alessandro Bosmani, Sandip Pujari, Laure Guenée, Céline Besnard, Amalia Isabel Poblador Bahamonde, Jérôme Lacour
Chem. Eur. J. 2017, 23, 8678-8684
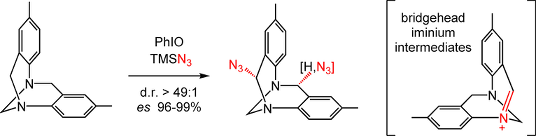
In the context to Tröger base chemistry, regio and stereoselective Csp3-H azidation reactions are reported. Azide functional groups are introduced at either one or the two benzylic positions selectively. Mild conditions and good yields are afforded by the combination of TMSN3 and iodosobenzene PhIO. The process occurs with high enantiospecificity (es 96-99%) and, interestingly and importantly, via bridgehead iminium intermediates as shown by mechanistic and in-silico studies. Finally, mono and bis triazole derivatives were prepared in high yields and enantiospecificity using CuAAC reactions; some of the products being used as anion-binding organocatalysts for the tritylation of amines and alcohols.
DOI : 10.1002/chem.201700845
archive ouverte unige:95273
