- Zhao, Y.; Cotelle, Y.; Avestro, A.-J.; Sakai, N.; Matile, S. “Asymmetric Anion-π Catalysis: Enamine Addition to Nitroolefins on π-Acidic Surfaces” J. Am. Chem. Soc. 2015, 137, 11582-11585
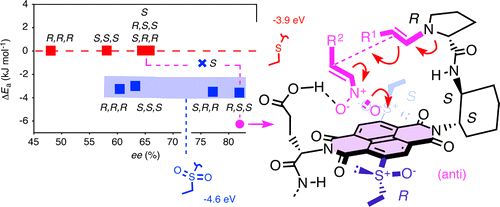
Here we provide experimental evidence for anion-π catalysis of enamine chemistry and for asymmetric anion-π catalysis. A proline for enamine formation on one side and a glutamic acid for nitronate protonation on the other side are placed to make the enamine addition to nitroolefins occur on the aromatic surface of π-acidic naphthalenediimides. With increasing π acidity of the formally trifunctional catalysts, rate and enantioselectivity of the reaction increase. Mismatched and more flexible controls reveal that the importance of rigidified, precisely sculpted architectures increases with increasing π acidity as well. The absolute configuration of stereogenic sulfoxide acceptors at the edge of the π-acidic surface has a profound influence on asymmetric anion-π catalysis and, if perfectly matched, affords the highest enantio- and diastereoselectivity.
DOI: 10.1021/jacs.5b07382
open archive unige:75233 • pdf ![]()
