- Morelli, P.; Martin-Benlloch, X.; Tessier, R.; Waser, J.; Sakai, N.; Matile, S. “Ethynyl Benziodoxolones: Functional Terminators for Cell-Penetrating Poly(disulfide)s” Polym. Chem. 2016, 7, 3465-3470
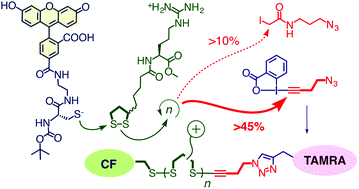
Outperforming cell-penetrating peptides (CPPs), cell-penetrating poly(disulfide)s (CPDs) are attracting increasing interest. CPDs are accessible by ring-opening disulfide-exchange polymerization under mild conditions in neutral water. Initiation of the polymerization with thiols results in quantitative labeling of one CPD terminus with initiators of free choice. In contrast, labeling of the other terminus with iodoacetamides has so far been ineffective because of poor yields and the high excess of reagents needed. In this report, we introduce hypervalent iodine reagents as operational terminators of the synthesis of CPDs, also at high dilution. The power of the approach is exemplified with green-fluorescent initiators and ethynyl benziodoxolone terminators containing additional azides for CuAAC with red-fluorescent alkynes. The absorption spectra of the resulting CPDs demonstrate that stoichiometric application of ethynyl benziodoxolone terminators results in 46% incorporation. FRET between green-fluorescent initiators and red-fluorescent terminators demonstrates significant folding of CPDs in solution; it disappears upon reductive depolymerization. Substrates attached to the new termini are shown to enter into HeLa cells. Moreover, disappearance of FRET in the cytosol corroborated the reductive cleavage of CPDs upon internalization. Beyond the introduction of enthynyl benziodoxolones as operational terminators, these findings thus demonstrate also the compatibility of CuAAC with poly(disulfide)s and the usefulness of doubly-labeled CPDs for structural and mechanistic studies.
DOI: 10.1039/C6PY00562D
open archive unige:83688 • pdf ![]()
