- Laurent, Q.; Sakai, N.; Matile, S. “The Opening of 1,2-Dithiolanes and 1,2-Diselenolanes: Regioselectivity, Rearrangements, and Consequences for Poly(disulfide)s, Cellular Uptake and Pyruvate Dehydrogenase Complexes” Helv. Chim. Acta 2019, 102, e1800209
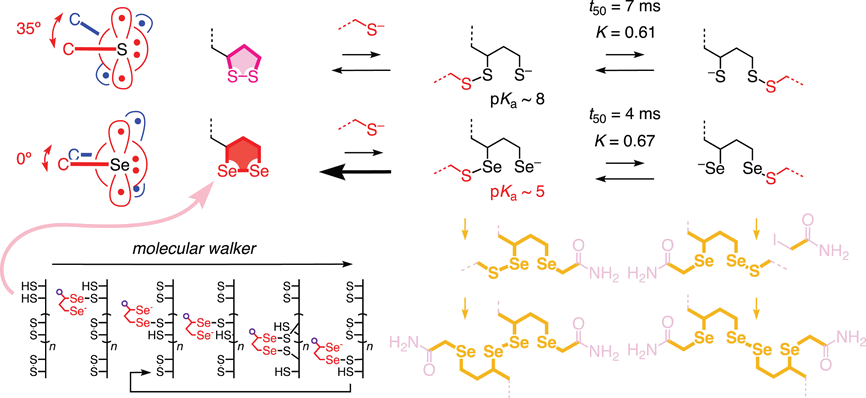
The thiol‐mediated opening of 3‐alkyl‐1,2‐dithiolanes and diselenolanes is described. The thiolate nucleophile is shown to react specifically with the secondary chalcogen atom, against steric demand, probably because the primary chalcogen atom provides a better leaving group. Once released, this primary chalcogen atom reacts with the obtained secondary dichalcogenide to produce the constitutional isomer. Thiolate migration to the primary dichalcogenide equilibrates within ca. 20 ms at room temperature at a 3 : 2 ratio in favor of the secondary dichalcogenide. The clarification of this focused question is important for the understanding of multifunctional poly(disulfide)s obtained by ring opening disulfide exchange polymerization of 3‐alkyl‐1,2‐dithiolanes, to rationalize the cellular uptake mediated by 3‐alkyl‐1,2‐diselenolanes as molecular walkers and, perhaps, also of the mode of action of pyruvate dehydrogenase complexes. The isolation of ring‐opened diselenolanes is particularly intriguing because dominant selenophilicity disfavors ring opening strongly.
open archive unige:114504 • pdf ![]()
