- Strakova, K.; Assies, L.; Goujon, A.; Piazzolla, F.; Humeniuk, H. V.; Matile, S. “Dithienothiophenes at Work: Access to Mechanosensitive Fluorescent Probes, Chalcogen Bonding Catalysis, and Beyond” Chem. Rev. 2019, 119, 10977-11005
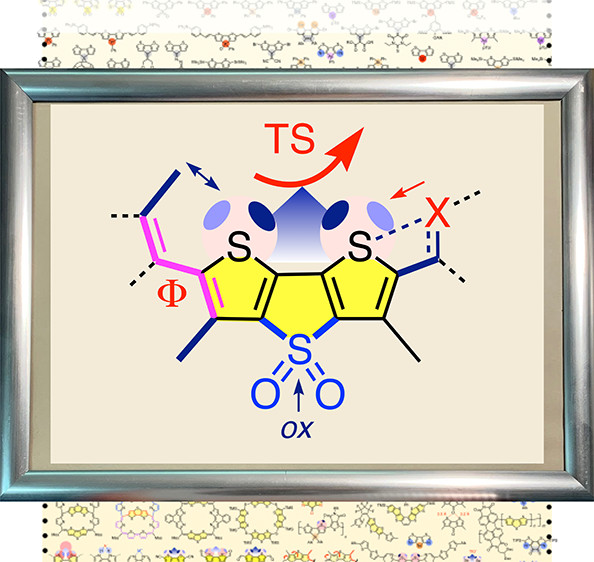
In this review, the multifunctionality of dithieno[3,2-b:2′,3′-d]thiophenes (DTTs) is covered comprehensively. This is of interest because all involved research is very recent and emphasizes timely topics such as mechanochemistry for bioimaging or chalcogen bonds for catalysis and solar cells and because the newly emerging privileged scaffold is embedded in an inspiring structural space. At the beginning, DTTs are introduced with regard to nomenclature, constitutional isomers, and optoelectronic properties. The structural space around DTTs is mapped out next with regard to heteroatom substitution in the bridge and core, covering much of the periodic table, eccentric heteroatom doping, and bridge expansions. After a brief summary of synthetic approaches to the DTT scaffold, chalcogen bonds are introduced as, together with redox switching and turn-on fluorescence, one of the three conceptual foundations of the most multifunctionality. Realized functions cover anion binding, transport (ion carriers, ion channels), catalysis, and the first fluorescent probes to image physical forces in living cells. The appearance of DTTs in many other photosystems covers push–pull systems for nonlinear optics and dye-sensitized solar cells, DTT polymers in light-emitting diodes, organic field-effect transistors and organic photovoltaics, DTT self-assembly and templated assembly into thin films and fluorescent fibers, also within cells, and the integration of DTTs into photochromes and biaromatics that violate the Hückel rule.
DOI: 10.1021/acs.chemrev.9b00279
open archive unige:124272 • pdf ![]()
