Liste
Précédente Suivante
Publication 65
- Direct Access to Chiral Secondary Amides by Copper-Catalyzed Borylative Carboxamidation of Vinylarenes with Isocyanates
Daniele Fiorito, Yangbin Liu, Celine Besnard, Clément Mazet*
J. Am. Chem. Soc. 2020, 142, 623-632
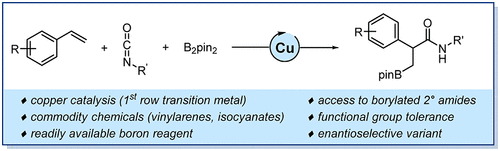
A Cu-catalyzed borylative carboxamidation reaction has been developed using vinylarenes and isocyanates. Alkynes, branched 1,3-dienes and bicyclic alkenes were also found to be competent coupling partners. Using a chiral phosphan-amine ligand, an enantioselective variant of this transformation was developed, affording a set of α-chiral amides with unprecedented levels of enantioselectivity. The synthetic utility of the method was demonstrated through a series of representative stereoretentive post-catalytic derivatizations.
archive ouverte unige:128621
DOI de la version éditeur : 10.1021/jacs.9b12297
DOI de l'AAM : 10.26037/yareta:2mil4dycf5ccdjxiaj7k7livtu
DOI du Dataset : 10.26037/yareta:pghrktzptzhrlcpykr25424mlq
