Publication 83
- Remote Functionalization by Pd-Catalyzed Isomerization of Alkynyl Alcohols
Simone Scaringi, Baptiste Leforestier, Clément Mazet*
J. Am. Chem. Soc. 2024, 146, 18606-18615
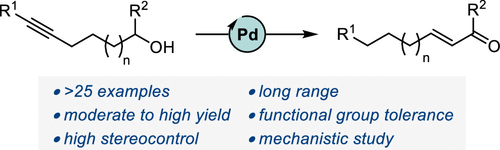
In recent years, progress has been made in the development of catalytic methods that allow remote functionalizations based on alkene isomerization. In contrast, protocols based on alkyne isomerization are comparatively rare. Herein, we report a general Pd-catalyzed long-range isomerization of alkynyl alcohols. Starting from aryl-, heteroaryl-, or alkyl-substituted precursors, the optimized system provides access preferentially to the thermodynamically more stable α,β-unsaturated aldehydes and is compatible with potentially sensitive functional groups. We showed that the migration of both π-components of the carbon–carbon triple bond can be sustained over several methylene units. Computational investigations served to shed light on the key elementary steps responsible for the reactivity and selectivity. These include an unorthodox phosphine-assisted deprotonation rather than a more conventional β-hydride elimination in the final tautomerization event.
DOI de la version éditeur : 10.1021/jacs.4c05136 (open access)
