Liste
Précédente Suivante
Publication 18
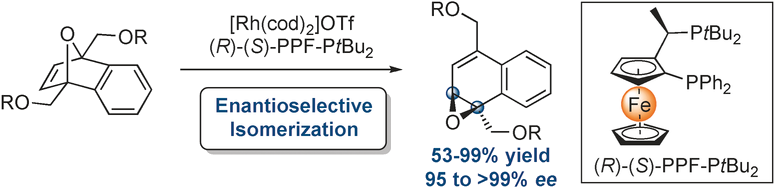
- Rhodium-Catalyzed Enantioselective Isomerization of meso-Oxabenzonorbornadienes to 1,2-Naphthalene Oxides
Yen, A.; Choo, K.-L.; Yazdi, S. K.; Franke, P. T.; Webster, R.; Franzoni, I.; Loh, C. C. J.; Poblador-Bahamonde, A. I.; Lautens, M.
Angew. Chem. Int. Ed. 2017, 56, 6307-6311

Herein we describe a rhodium-catalyzed enantioselective isomerization of meso-oxabicyclic alkenes to 1,2-naphthalene oxides. These potentially useful building blocks can be accessed in moderate to excellent yields with impressive enantioselectivities. Additionally, experimental findings supported by preliminary computations suggest that ring-opening reactions of bridgehead disubstituted oxabicyclic alkenes proceed through the intermediacy of these epoxides and may point to a kinetically and thermodynamically favored reductive elimination as the origin for the observed enantioselectivities.
DOI : 10.1002/anie.201700632
archive ouverte unige:94490
