Liste
Précédente Suivante
Publication 177
-
P. Müller, S. Grass, S.P. Shahi, G. Bernardinelli,
“Rh(II)-Catalyzed asymmetric carbene transfer with ethyl 3,3,3-trifluoro-2-diazopropionate”
Tetrahedron 2004, 60, 4755-4763.
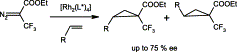
The asymmetric cyclopropanation of 1,1-diphenylethylene (2) with ethyl 3,3,3-trifluoro-2-diazopropionate (1) in the presence of chiral Rh(II) catalysts affords cyclopropane 3 with yields and enantioselectivities of up to 72 and 40%, respectively. Similar results are obtained for asymmetric cyclopropenation of hex-1-yne (4), although enantioselectivity is lower. The cyclopropanation of mono-substituted olefins (8a–8e) with 1 leads to cis/trans-mixtures of cyclopropanes 9a–9e with a maximum ee of 75% for 4-methoxystyrene (8c).
