Liste
Précédente Suivante
Publication 180
-
P. Müller, A. Ghanem,
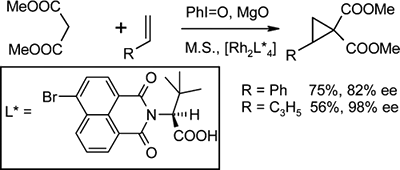
“Rh(II)-Catalyzed Enantioselective Cyclopropanation of Olefins with Dimethyl Malonate via in Situ Generated Phenyliodonium Ylide”
Org. Lett. 2004, 6, 4347-4350.

Olefins are cyclopropanated with dimethyl malonate (1a) iodosylbenzene (PhIO) and a Rh(II) carboxylate catalyst via an in situ generated phenyliodonium ylide (1c). Enantioselectivities of up to 90% for 4-bromostyrene and 98% for pent-1-ene have been observed with (S)-N-4-bromo-1,8-naphthanoyl-tert-leucine (4c) as the chiral ligand. The same catalyst was effective for olefin cyclopropanation with Meldrum's acid, giving cyclopropanes with 96% (with styrene) and 87% ee (with pent-1-ene), respectively.
DOI : 10.1021/ol048159u
