- Access to 1,2- and 1,3-Amino Alcohols via Cu-Catalyzed Enantioselective Borylations of Allylamines
Flaget, A.; Mazet, C.
Org. Lett. 2024, 26, 8542-8547
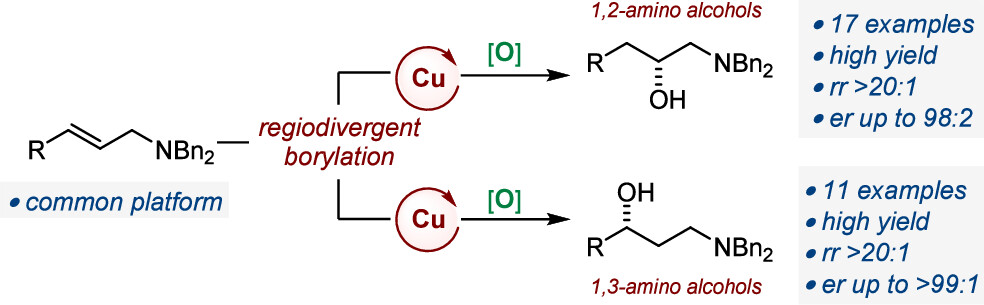
We report Cu-catalyzed enantioselective borylation/oxidation sequences that afford either enantioenriched 1,2- or 1,3-amino alcohols starting from N,N-protected allylamines as a common platform. The approach is based on the mechanistic specificities of catalytic hydroboration and protoboration reactions, which formally install a boron unit and a hydrogen atom at opposite positions of a C?C bond. The nature of the substituents of the carbon–carbon double bond also exerts a determining influence on the regioselectivity of insertion of the catalytically active Cu species. Both protocols are regio- and enantioselective and applicable to a broad range of substrates. Homoallylamines are also competent substrates, providing access to 1,3- and 1,4-amino alcohols.
