Intérêts de recherche (en anglais)
Asymmetric synthesis and methodologies by using both metal (particularly copper reagents) and nonmetallic catalysts (organocatalysis); Chiral bases and chiral protonation reagents; Development of new reactions and new methodologies for organic synthesis; Design of new chiral ligands, particularly the ones derived from C2 symmetrical diamines and diols; Application to the synthesis of natural products.
Asymmetric synthesis in a wide sense.
Catalytic organocopper chemistry from the asymmetric point of view.
Chiral bases and chiral protonation reagents.
Development of new reactions and new methodologies for organic synthesis.
New Organocatalysed reactions and organocatalysts.
Design of new chiral ligands, particularly the ones derived from C2 symmetrical diamines and diols.
Application to the synthesis of natural products.
Organocatalysis
Design of new powerful catalysts:
|
|
iPBP
|
Aminal-Pyrrolidines
|
Application of new nucleophiles / electrophiles for the synthesis of valuable synthons:
Nucleophiles: |
|
Electrophiles: |
|
Combination of organometallic and organocatalysis for the generation of high molecular complexity:
Au-Organocatalysis: |
|
Cu-Organocatalysis: |
|
Ir-Organocatalysis: |
|
Related publications:
Catalyst design: Eur. J. Org. Chem.
2010, 927-936 • Chem. Eur. J.
2008, 14, 7504-7507 • Adv. Synth. Cat.
2004, 346, 1147-1168 • Org. Lett.
2002, 4, 3611-3614.
New nucleophiles / electrophiles: Chem. Commun.
2010, 46, 4085-4087 • Tetrahedron: Asymmetry
2010, 21, 1666-1673 • Chem. Eur. J.
2009, 15, 11109-11113 • Org. Lett.
2008, 10, 4557-4560 • Chem. Commun.
2008, 4694-4696 • Org. Lett.
2007, 9, 3749-3752 • Org. Lett.
2005, 7, 4361-4364 • Org. Lett.
2003, 5, 2559-2561.
Metal-organocatalysis combination: Adv. Synth. Catal.
2010, 352,1856-1860 • Adv. Synth. Catal.
2010, 352,667-695 • Angew. Chem. Int. Ed.
2009, 48, 8923-8926.
Ring opening of O or N bridge
- Ring Opening of meso Epoxides:

- Ring Opening of Cyclic Vinyl Epoxides:


- Ring Opening of meso bicyclic hydrazines:

- Ring Opening of Oxabenzonorbornadienes:
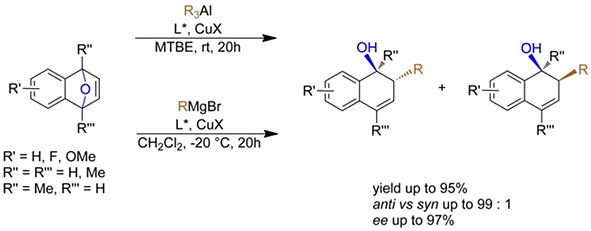

- Ring Opening Cyclic Alkenyl Cyclopropane Malonates:

Related Publications:
Org. Lett. 2010, 12, 576-579 •
Synlett 2010, 317-320 •
Eur. J. Org. Chem. 2009, 4949-4955 •
Synthesis 2009, 2101-2112 •
Tetrahedron Lett. 2009, 50, 3474-3477 •
Synlett 2008, 12, 1797-1800 •
Synlett 2007, 435-438 •
Org. Lett. 2006, 8, 3581-3584 •
Eur. J. Org. Chem. 2005, 1354-1366 •
In Methodologies in Asymmetric Catalysis, S. V. Malhotra, Ed.; ACS Symposium Series, Volume 880, Washington, 2004, 43-59 •
Tetrahedron: Asymmetry 2004, 15, 1531-1536 •
Eur. J. Org. Chem. 2004, 2151-2159 •
Tetrahedron: Asymmetry 2004, 15, 1069-1072 •
J. Chem. Soc., Perkin Trans. 1 2000, 3354-3355 •
J. Chem. Soc., Perkin Trans. 1 2000, 3352-3353 •
Synlett 1998, 1165-1167.
Asymmetric Conjugate Addition
- Creation of quaternary stereogenic centres:
Till the first publication of creation of quaternary stereogenic centres via copper catalyzed conjugate asymmetric addition (ACIE, 2005, 1376-1378) great research efforts have been done to extend the scope of nucleophiles and substrates in this area.
- Scope of nucleophiles used for Cu-catalyzed ACA:
Alkyl nucleophiles:

M. d'Augustin, L. Palais, A. Alexakis,
Angew Chem. Int. Ed. 2005, 44, 1376-1378.

D. Martin, S. Kehrli, M. d'Augustin, H. Clavier, M. Mauduit, A. Alexakis,
J. Am. Chem. Soc. 2006, 128, 8416-8417.

H. Hénon, M. Mauduit, A. Alexakis,
Angew. Chem. Int. Ed. 2008, 47, 9122-9124.
Alkenyl nucleophiles:
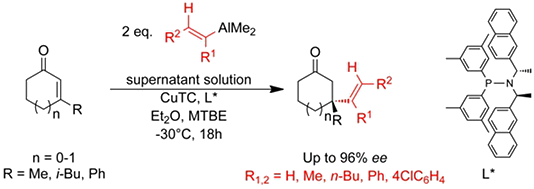
D. Müller, C. Hawner, M. Tissot, L. Palais, A. Alexakis,
Synlett 2010, 1694-1698.
Aryl nucleophiles:

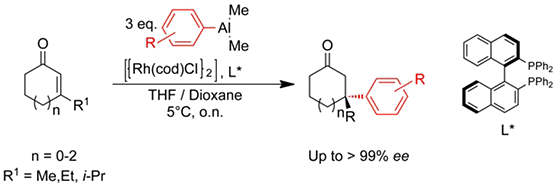
C. Hawner, K. Li, V. Cirriez, A. Alexakis,
Angew. Chem. Int. Ed. 2008, 47, 8211-8214.
C. Hawner, D. Müller, L. Gremaud, A. W. Felouat, S. Woodward, A. Alexakis,
Angew Chem. Int. Ed. 2010, 122, 7769-7772.
- "New" substrates for the copper catalyzed ACA:
New original and challenging substrates have also been applied in copper catalysis.

L. Palais, L. Babel, A. Quintard, S. Belot, A. Alexakis,
Org. Lett. 2010, 12, 1988-1991.
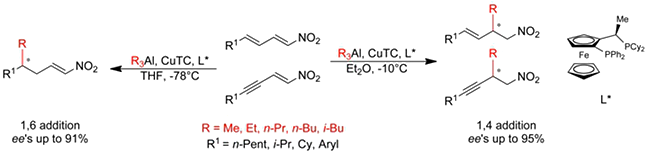
M. Tissot, D. Müller, S. Belot, A. Alexakis,
Org. Lett. 2010, 12, 2770-2773.
Asymmetric Br-Li Exchange
- Design of new ligand:

- Brome-Lithium exchange catalyzed by ether diamines or diether derivatives:

Related publications:
Tetrahedron: Asymmetry 2009, 20, 1004-1007 • Adv. Synth. Catal. 2010, 352, 2611-2620.