Liste
Précédente Suivante
Publication 141
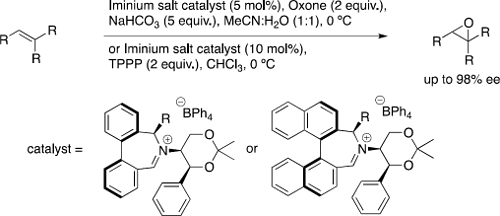
- Asymmetric Epoxidation Using Iminium Salt Organocatalysts Featuring Dynamically Controlled Atropoisomerism
Philip Charles Bulman Page, Christopher James Bartlett, Yohan Chan, David Day, Phillip Parker, Benjamin R Buckley, Geracimos A. Rassias, Alexandra M. Z. Slawin, Steven M. Allin, Jérôme Lacour, André Pinto
J. Org. Chem. 2012, 77, 6128-6138

Introduction of a pseudoaxial substituent at a stereogenic centre adjacent to the nitrogen atom in binaphthyl- and biphenyl- derived azepinium salt organocatalysts affords improved enantioselectivities and yields in the epoxidation of unfunctionalized alkenes. In the biphenyl-derived catalysts, the atropoisomerism at the biphenyl axis is controlled by the interaction of this substituent with the chiral substituent at nitrogen.
DOI : 10.1021/jo300915u
archive ouverte unige:21933
