Publication 199
- Configurational Lability of Imino-Substituted Ethano Tröger Bases. Insight on the Racemization Mechanism
Alessandro Bosmani, Alejandro Guarnieri-Ibáñez, Jérôme Lacour
Helv. Chim. Acta 2019, 102, e1900021
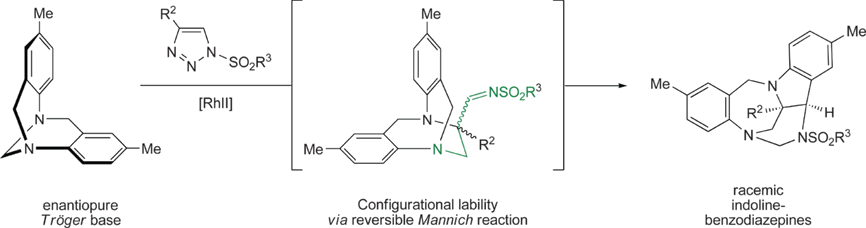
Polycyclic indoline‐benzodiazepines are afforded in one step by the reaction of Tröger bases with N‐sulfonyl‐1,2,3‐triazoles under Rh(II) catalysis. After α‐imino carbene formation, the process involves a cascade of [1,2]‐Stevens rearrangement, Friedel‐Crafts, Grob fragmentation and aminal formation reactions. It is highly diastereoselective (d.r.>49:1, four stereocenters incl. two bridgehead N atoms). However and in contrast with other reported carbene additions to these moieties, full racemization occurs when enantiopure Tröger Bases are used as substrates. To pinpoint the origin of this unexpected behavior, key elemental steps of the mechanism were evaluated and tested. Interestingly, it is not only the initial ring‐opening but also the latter reversible Mannich reaction of the imino‐substituted ethano Tröger base intermediate that is responsible for the loss of enantiospecificity.
DOI : 10.1002/hlca.201900021
archive ouverte unige:115269
