Liste
Précédente Suivante
Publication 217
- Chiral Near-Infrared Fluorophores by Self-Promoted Oxidative Coupling of Cationic Helicenes with Amines/Enamines
Johann Bosson, Geraldine M. Labrador, Céline Besnard, Denis Jacquemin, Jérôme Lacour
Angew. Chem. Int. Ed. 2021, 60, 8733-8738

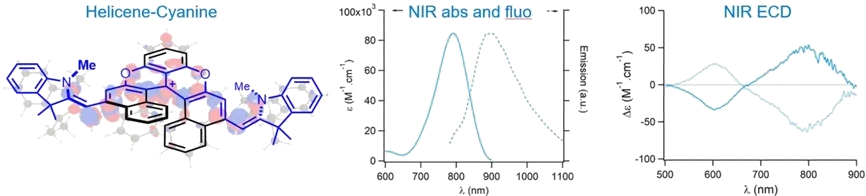
In one pot, tertiary alkyl amines are oxidized to enamines by cationic dioxa [6]helicene, which further reacts as electrophile and oxidant to form mono or bis donor-π-acceptor coupling products. This original and convergent synthetic approach provides a strong extension of conjugation yielding chromophores that absorb intensively in far-red or NIR domains (λmax up to 791 nm) and fluoresce in the NIR as well (λmax up to 887 nm). Intense ECD properties around 790 nm with a |Δε| value up to 60 M-1 cm-1 are observed.
DOI : 10.1002/anie.202016643
archive ouverte unige:150867
