Liste
Précédente Suivante
Publication 224
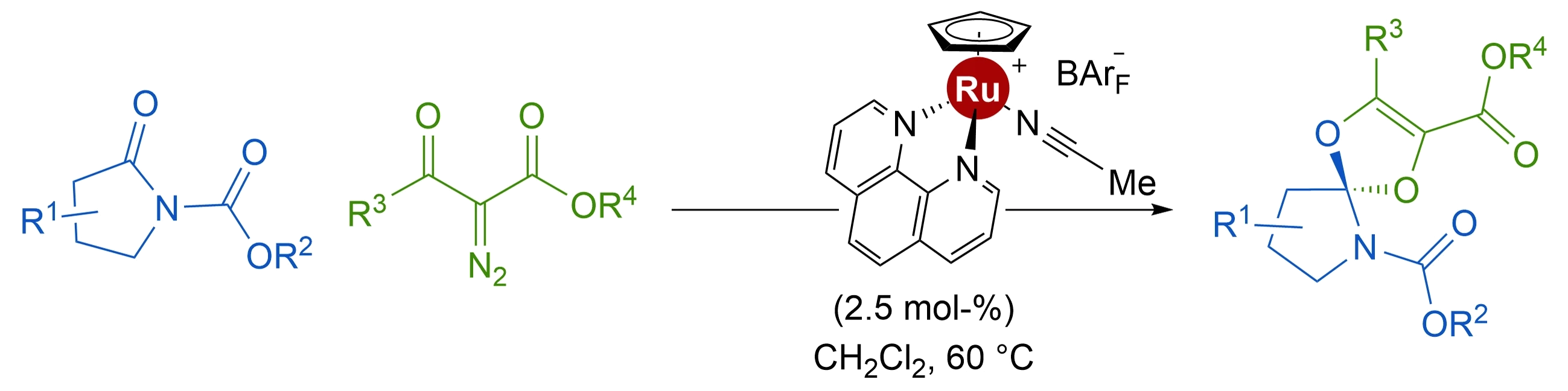
- Spirocyclic Amide Acetal Synthesis by [CpRu]-Catalyzed Condensations of α-Diazo-β-Ketoesters with γ-Lactams
Romain Pertschi, Elodie Brun, Adiran De Aguirre Fondevila, Laure Guénée, Amalia I. Poblador-Bahamonde, Jérôme Lacour
Helv. Chim. Acta 2021, 104, e2100122

The synthesis of spirocyclic amide acetals (33-93%) has been achieved through Ru(II)-catalyzed condensations of N-carbamate protected pyrrolidinones with metal carbenes derived from α-diazo-β-ketoesters. Thanks to the mildness of the diazo decomposition conditions induced by a 1:1 combination of [CpRu(MeCN)3][BArF] and 1,10-phenanthroline, the formation of the sensitive products is possible. Full characterization of this carbonyl-ylide mediated process is provided by DFT calculations.
Dedicated to Prof. Peter Kündig on the occasion of his 75th birthday.
DOI : 10.1002/hlca.202100122
archive ouverte unige:155554
