Publication 227
- Acetylene Derivatives of Cationic Diazaoxatriangulenes and Diaza [4]Helicenes – Access to Red Emitters and Planar Chiral Stereochemical Traits
Pavol Ondrisek, Margaux Elie, Marion Pupier, Adiran de Aguirre, Amalia I. Poblador Bahamonde, Céline Besnard, Jérôme Lacour
Chem. Eur. J. 2022, 28, e202104405
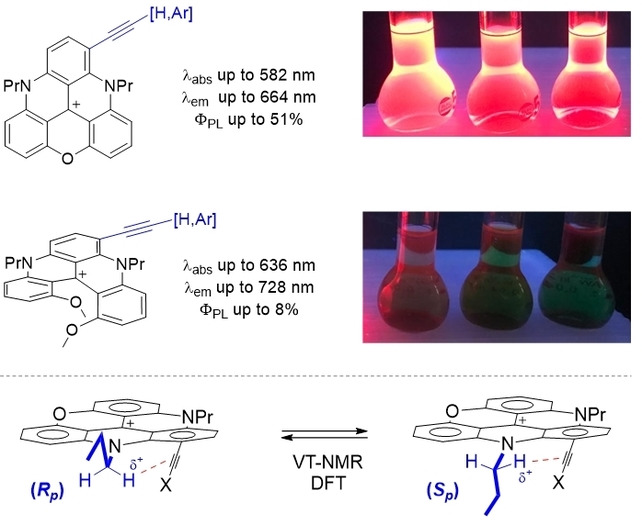
Cationic triangulenes, and related helicenes, constitute a rich class of dyes and fluorophores, usually absorbing and emitting light at low energy, in the orange to red domains. Recently, to broaden the scope of applications, regioselective late-stage functionalizations on these core moieties have been developed. For instance, with the introduction of electron-donating groups (EDGs), important bathochromic shifts are observed pushing absorptions towards or in the near-infrared (NIR) spectral domain while emissive properties disappear essentially completely. Herein, to upset this drawback, acetylene derivatives of cationic diazaoxa triangulenes (DAOTA) and [4]helicenes are prepared (16 examples). Contrary to other EDG-functionalized derivatives, C≡C-functionalized products remain broadly fluorescent, with red-shifted absorptions (Δλabs up to 25 nm) and emissions (Δλem up to 73 nm, ΦPL up to 51%). Quite interestingly, a general dynamic stereoisomerism phenomenon is evidenced for the compounds derived from achiral DAOTA cores. At low temperature in 1H-NMR spectroscopy (218 K), N-CH2 protons become diastereotopic with chemical shifts differences (Δδ) as high as +1.64 ppm. The signal coalescence occurs around 273 K with a barrier of ~12 kcal.mol-1. This phenomenon is due to planar chiral conformations (Sp and Rp configurations), induced by the geometry of the alkyl (n-propyl) side-chains next to the acetylenic substituents. Finally, DFT calculations offer a valuable insight on geometries, stereodynamics and on the large difference in NMR for some of the diastereotopic protons.
DOI : 10.1002/chem.202104405
archive ouverte unige:159531
