Publication 240
- 2,4,5,7-Tetranitrofluorenone Oximate for the Naked-Eye Detection of H-Bond Donors and the Chiroptical Sensing of Enantiopure Reagents
Dávid Pál, Céline Besnard, Adiran de Aguirre, Amalia I. Poblador-Bahamonde, Gennaro Pescitelli, Jérôme Lacour
Chem. Eur. J. 2023, 29, e202302169
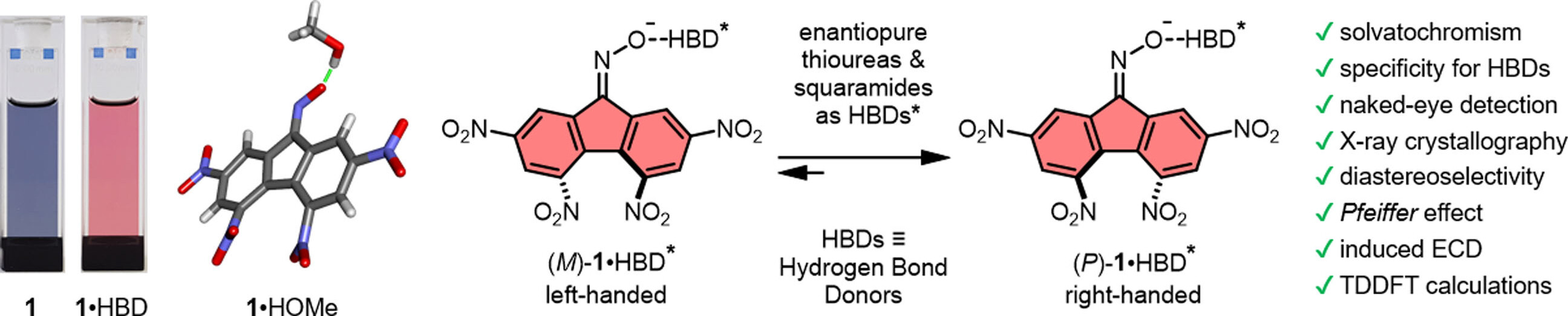
Hydrogen bonding greatly influences rates and equilibrium positions of chemical reactions, conformations, and sometimes even stereochemistry. Herein, we report on tetranitrofluorenone oximate, a novel dye capable of naked-eye detection of hydrogen-bond donating species (HBDs) and of rapid determination of H-bond donation strength by hypsochromic shift monitoring. In addition, the molecule possesses atropisomeric conformations, of M and P configuration, as evidenced in solid and solution state studies by X-ray diffraction and electronic circular dichroism (ECD), respectively. In the latter case, enantiopure bis-thioureas were the most effective HBDs to promote a chiral induction (diastereoselective recognition, Pfeiffer effect); the ECD results being rationalized by time-dependent density functional theory (TDDFT) calculations. Based on these experiments, bis-thioureas were used as chiral reagents in asymmetric 1,3-dipolar cycloadditions of structurally-related nitrones; the ECD sensing of the stereoinduction between bis-thioureas and the oximate serving as an indirect method of selection of the most effective HBD for asymmetric synthesis.
DOI : 10.1002/chem.202302169
archive ouverte unige:174444
