- Alonso Doval, D.; Dal Molin, M.; Ward, S.; Fin, A.; Sakai, N.; Matile, S. “Planarizable Push-Pull Oligothiophenes: In Search of the Perfect Twist” Chem. Sci. 2014, 5, 2819-2825
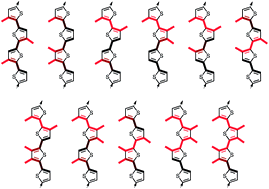
The concept to couple fluorophore planarization and fluorophore polarization for the construction of innovative fluorescent membrane probes is elaborated comprehensively in the context of oligothiophenes. Increasing length with different degree of twist from ter- to quinquethiophenes results in increasing extinction coefficients, decreasing quantum yields and relatively minor red shifts. Quaterthiophenes show maximal Stokes shifts and are thus preserved to further elaborate on deplanarization. Increasing quaterthiophene deplanarization results in increasing blue shifts and decreasing quantum yields in solution, whereas planarization in solid-ordered lipid bilayer membranes gives the respective red shifts with fluorescence recovery. An extensive screening reveals that intermediate global deplanarization with strong individual twists near the membrane interface are best. Weaker and stronger global twisting and strong individual twists deeper in the membrane are less convincing because planarization becomes either too easy or too difficult. The best probe reports decreasing membrane fluidity with a red shift of 44 nm and a fluorescence increase of almost 500%. These insights are important because they cover significant chemical space to help improving our understanding of chromophore twisting and promise bright perspectives with regard to biological applications and refined probe design.
DOI: 10.1039/C4SC00939H
open archive unige:37230 • pdf ![]()
