- Verolet, Q.; Rosspeintner, A.; Soleimanpour, S.; Sakai, N.; Vauthey, E.; Matile, S. “Turn-On Sulfide π Donors: An Ultrafast Push for Twisted Mechanophores” J. Am. Chem. Soc. 2015, 137, 15644-15647
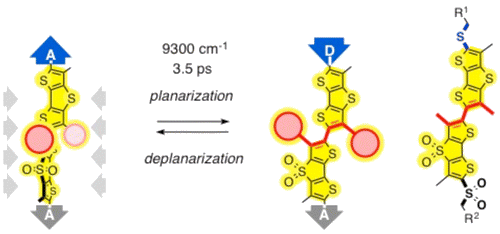
Attached to electron-rich aromatic systems, sulfides are very weak acceptors; however, attached to electron-poor aromatics, they turn into quite strong donors. Here, we show that this underappreciated dual nature of sulfides deserves full consideration for the design of functional systems. Tested with newly designed and synthesized planarizable push−pull mechanophores, sulfide acceptors in the twisted ground state are shown to prevent oxidative degradation and promote blue-shifting deplanarization. Turned on in the planar excited state, sulfide donors promote red-shifting polarization. Impressive Stokes shifts are the result. Demonstrating the usefulness of time-resolved broadband emission spectra to address significant questions, direct experimental evidence for the ultrafast (3.5 ps), polarity-independent and viscosity-dependent planarization from the twisted Franck−Condon S1 state to the relaxed S1 state could be secured.
DOI: 10.1021/jacs.5b10879
open archive unige:79049 • pdf ![]()
