- Akamatsu, M.; Matile, S. “Expanded Chiral Surfaces for Asymmetric Anion-π Catalysis” Synlett 2016, 27, 1041-1046
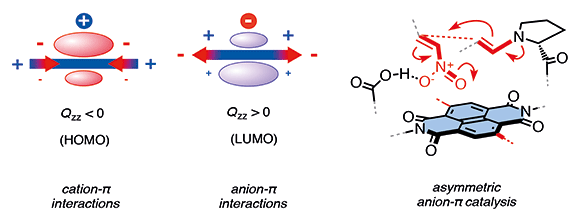
The insertion of a π-acidic surface of a naphthalenediimide (NDI) between a proline and a glutamate group affords trifunctional catalysts for the stereoselective addition of aldehydes to nitroolefins. In this report, phenyl sulfides are added to this central NDI surface. Oxidation of the sulfide donors into sulfoxide and sulfone acceptors increases both rate and stereoselectivity of the reaction. This dependence on π acidity provides corroborative support that anion–π interactions can contribute to asymmetric catalysis. Non-planar π surfaces around chiral sulfoxide connectors have a profound impact on stereoselectivity. Anti stereoisomers, with phenyl wings pointing in opposite directions from the central NDI surface, perform best in chloroform/methanol mixtures. With stronger anion–π interactions in more hydrophobic aromatic solvents, this trend inverts. Catalysis within π-box binding pockets between the two phenyl wings in syn architectures gives better selectivity under these conditions. The best results are obtained in toluene, whereas competitive π–π interactions with aromatic solvents of varied π acidity reduce the stereoselectivity. Diastereoselectivities up to 96% and enantiomeric excess values up to 91% with expanded surfaces exceed the performance of the original anion–π catalysts with identical chiral architecture (64% ee under identical conditions) and enters into the range of the best conventional catalysts.
open archive unige:83528 • pdf ![]()
