- Benz, S.; Mareda, J.; Besnard, C.; Sakai, N.; Matile, S. “Catalysis with Chalcogen Bonds: Neutral Benzodiselenazole Scaffolds with High-Precision Selenium Donors of Variable Strength” Chem. Sci. 2017, 8, 8164-8169
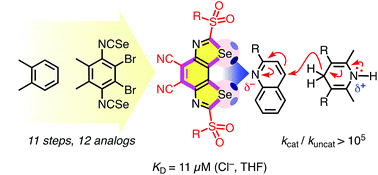
The benzodiselenazoles (BDS) introduced in this report fulfill, for the first time, all the prerequisites for non-covalent high-precision chalcogen-bonding catalysis in the focal point of conformationally immobilized σ holes on strong selenium donors in a neutral scaffold. Rational bite-angle adjustment to the long Se–C bonds was the key for BDS design. For the unprecedented BDS motif, synthesis of 12 analogs from o-xylene, crystal structure, σ hole variation strategies, optoelectronic properties, theoretical and experimental anion binding as well as catalytic activity are reported. Chloride binding increases with the depth of the σ holes down to KD = 11 μM in THF. Catalytic activities follow the same trend and culminate in rate enhancements for transfer hydrogenation of quinolines beyond 100 000.
DOI: 10.1039/C7SC03866F
open archive unige:99706 • pdf ![]()
