- Lim, B.; Cheng, Y.; Kato, T.; Pham, A.-T.; Le Du, E.; Mishra, A. K.; Grinhagena, E.; Moreau, D.; Sakai, N.; Waser, J.; Matile, S. “Inhibition of Thiol-Mediated Uptake with Irreversible Covalent Inhibitors” Helv. Chim. Acta 2021, 104, e2100085
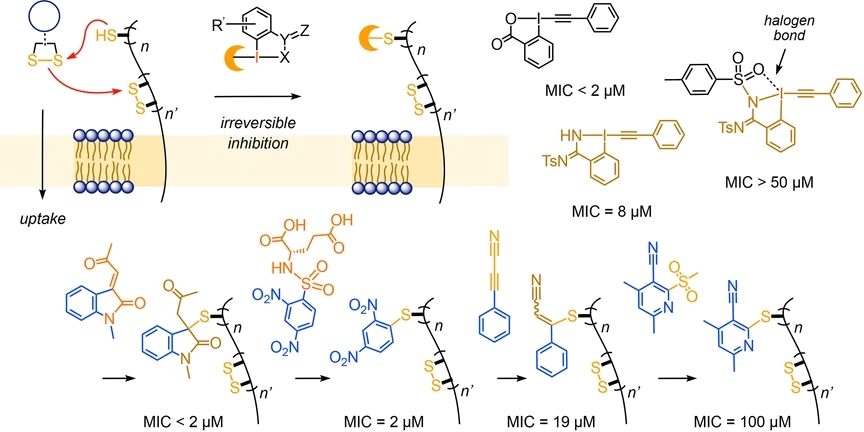
Thiol-mediated uptake is emerging as method of choice to penetrate cells. This study focuses on irreversible covalent inhibitors of thiol-mediated uptake. High-content high-throughput screening of the so far largest collection of hypervalent iodine reagents affords inhibitors that are more than 250?times more active than Ellman’s reagent and rival the best dynamic covalent inhibitors. Comparison with other irreversible reagents reveals that inhibition within one series follows reactivity, whereas inhibition across series deviates from reactivity. These trends support that molecular recognition, besides dynamic covalent exchange, contributes significantly to thiol-mediated uptake. The most powerful inhibitors besides the best hypervalent iodine reagents were Fukuyama’s nosyl protecting group and super-cinnamaldehydes that have been introduced as irreversible activators of the pain receptor TRPA1. Considering that several viruses use different forms of thiol-mediated uptake to enter cells, the identification of new irreversible inhibitors of thiol-mediated uptake is of general interest for the discovery of new antivirals.
Dedicated to Peter Kündig on the occasion of his 75th birthday.
open archive unige:153636 • pdf ![]()
