Publication 225
-
K. Li, A. Alexakis,
“Copper-Catalyzed Enantioselective Intramolecular Conjugate Addition/Trapping Reactions: Synthesis of Cyclic Compounds with Multichiral Centers”
Chem. Eur. J. 2007, 13, 3765-3771.
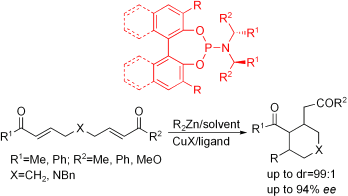
The Cu-catalyzed enantioselective conjugate addition of dialkylzinc to bis-α,β-unsaturated carbonyl compounds followed by the intramolecular trapping of the zinc enolate in the presence of chiral phosphoramidite ligands was investigated. Cyclic and heterocyclic compounds with multichiral centers were formed as a mixture of two diastereomers with excellent diastereoselectivities (up to 99:1) and enantioselectivities (up to 94 % ee,ee=enantiomeric excess). The stereochemistry was determined to be trans,trans for the major products and trans,cis for the minor products. The absolute configuration of the cyclic compounds was assigned by comparison with the analogous adduct derived fromtrans-3-nonen-2-one and Et2Zn or the adduct obtained through conjugate addition of Me2Zn to trans-1-phenyl-non-2-en-1-one. Further transformation of these cyclic products into more complex compounds is under investigation in our laboratory.
DOI : 10.1002/chem.200601327
archive ouverte unige:8126
