Publication 259
-
C. Ladjel, N. Fuchs, J. Zhao, G. Bernardinelli, A. Alexakis,
“New Bifunctional Substrates for Copper-Catalyzed Asymmetric Conjugate Addition Reactions with Trialkylaluminium”
Eur. J. Org. Chem. 2009, 4949-4955.
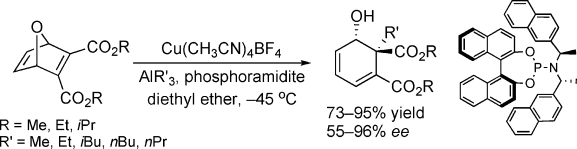
Trialkylaluminium reagents have been found to undergo a copper-catalyzed asymmetric conjugate addition (ACA) reaction with oxabicyclo[2.2.1]hepta-2,5-diene-2,3-dicarboxylates with the simultaneous creation of two stereocenters. Different types of ligands were tested, and chiral phosphoramidite ligands allowed the reaction to proceed with good yields and good to excellent enantioselectivity. Thesyn substitution product observed by X-ray analysis suggests an exo attack of the nucleophile. Herein we report the results obtained in the development of this copper-catalyzed ACA methodology, which affords an all-carbon quaternary center with unsubstituted and substituted oxacyclic Michael acceptors. The study was completed by mechanistic investigations performed to elucidate the original reaction pathway.
DOI : 10.1002/ejoc.200900662
archive ouverte unige:8235
