Liste
Précédente Suivante
Publication 308
-
F. Romanov-Michailidis, C. Besnard, A. Alexakis,
“N-Heterocyclic Carbene-Catalyzed Annulation of α-Cyano-1,4-diketones with Ynals”
Org. Lett. 2012, 14, 4906-4909.

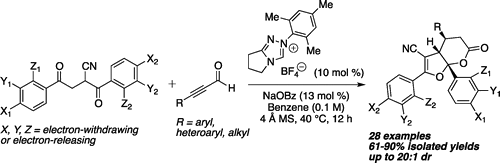
In this paper, the first stereoselective annulation reaction between α-cyano-1,4-diketones and ynals, mediated by catalytic amounts of a triazolium salt precatalyst and cocatalytic amounts of a weak carboxylate base, is disclosed. The title transformation proceeds smoothly under mild reaction conditions and generates three contiguous stereogenic centers, one of which is a quaternary acetal carbon. This reaction tolerates a wide variety of electronically distinct substituents on both reaction partners and affords privileged bicyclic scaffolds in 61–90% isolated yields and with up to 20:1 diastereomeric preference.
DOI : 10.1021/ol3022287
archive ouverte unige:23520
