Liste
Précédente Suivante
Publication 316
-
C. Bleschke, M. Tissot, D. Müller, A. Alexakis,
“Direct Trapping of Sterically Encumbered Aluminum Enolates”
Org. Lett. 2013, 15, 2152-2155.

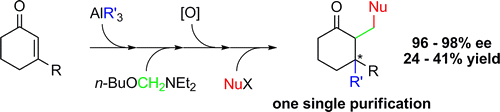
The formation of chiral and sterically congested cyclohexanone derivatives has been achieved through a multistep sequence with one single purification step. (n-Butoxymethyl)-diethylamine was identified as a highly efficient reagent for the direct trapping of aluminum enolates. The Lewis acidic character of aluminum suffices to activate the α-aminoether to form in situ an electrophilic iminium species. In return the aluminum enolate is rendered more nucleophilic by coordination of the butoxy group and formation of an aluminate.
DOI : 10.1021/ol400642y
archive ouverte unige:27733
