Liste
Précédente Suivante
Publication 319
-
F. Romanov-Michailidis, L. Guénée, A. Alexakis,
“Enantioselective Organocatalytic Fluorination-Induced Wagner-Meerwein Rearrangement”
Angew. Chem. Int. Ed. 2013, 52, 9266-9270.

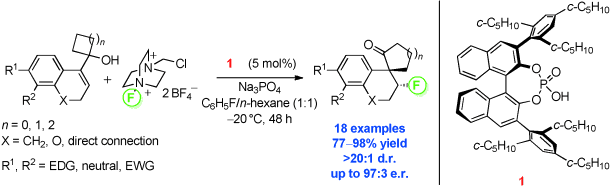
Cracked under strain: Strained allylic cyclobutanols and cyclopropanols readily undergo a ring expansion described by the title rearrangement. This reaction is promoted by catalytic amounts of 1 and displays high tolerance with respect to the substrate scope. The corresponding β-fluoro spiroketone products are isolated in high yields and with excellent stereoselectivities. EDG=electron-donating group, EWG=electron-withdrawing group.
DOI : 10.1002/anie.201303527
archive ouverte unige:29563
