Liste
Précédente Suivante
Publication 337
-
F. Romanov-Michailidis, M. Pupier, C. Besnard, T. Bürgi, A. Alexakis,
“Enantioselective Catalytic Fluorinative Aza-semipinacol Rearrangement”
Org. Lett. 2014, 16, 4988-4991.

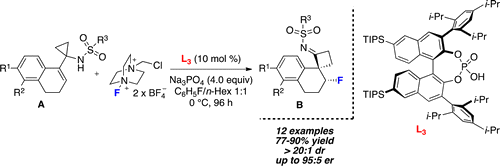
An efficient and highly stereoselective fluorinative aza-semipinacol rearrangement is described. The catalytic reaction requires use of Selectfluor in combination with the chiral, enantiopure phosphate anion derived from acid L3. Under optimized conditions, cyclopropylamines A were transformed into β-fluoro cyclobutylimines B in good yields and high levels of diastereo- and enantiocontrol. Furthermore, the optically active cyclobutylimines were reduced diastereoselectively with L-Selectride in the corresponding fluorinated amines C, compounds of significant interest in the pharmacological industry.
DOI : 10.1021/ol5022355
archive ouverte unige:41312
