Liste
Précédente Suivante
Publication 347
-
P. Cottet, C. Bleschke, M.G. Capdevila, M. Tissot, A. Alexakis,
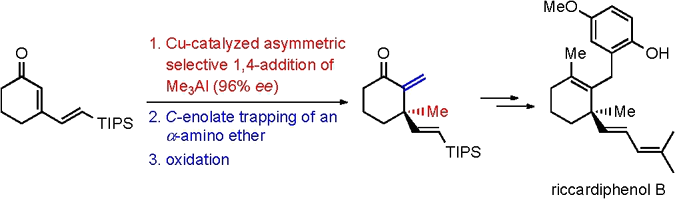
“Catalytic Enantioselective Total Synthesis of Riccardiphenol B”
Adv. Synth. Catal. 2016, 358, 417-425.

The first catalytic enantioselective total synthesis of riccardiphenol B, a sesquiterpene derivative isolated from a Japanese collection of the liverwort Riccardia crassa, has been achieved. A copper-catalyzed asymmetric conjugate addition of trimethylaluminum was used at an early stage to generate the quaternary stereogenic center with high enantiomeric excess. The corresponding sterically encumbered aluminum enolate was directly trapped with an α-amino ether, allowing after oxidation, the release of a key intermediate in the total synthesis of the target natural product.
DOI : 10.1002/adsc.201500928
archive ouverte unige:80311
