Publication 348
-
S. Goncalves-Contal, L. Gremaud, L. Palais, L. Babel, A. Alexakis,
“Copper-Catalyzed Enantioselective Conjugate Addition to α,β-Unsaturated Aldehydes with Various Organometallic Reagents”
Synthesis 2016, 48, 3301-3308.
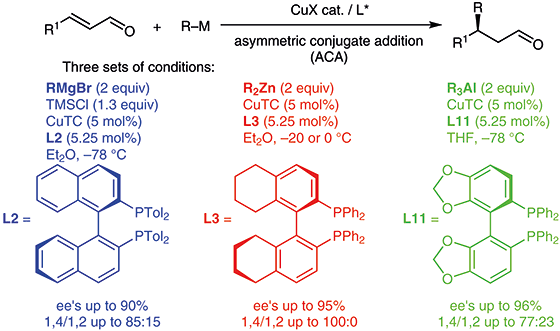
β-Substituted aldehydes constitute a very important class of compounds found in nature. Synthesis of this motif can be envisioned by C–C bond formation on enals. For this purpose, we report herein the development of enantioselective copper-catalyzed conjugate addition of various organometallic reagents to α,β-unsaturated aldehydes with (R)-H8BINAP, (R)-TolBINAP, and (R)-SEGPHOS as chiral ligands. Three sets of conditions were successfully developed and several enals were used. Reactivity and regio- and enantioselectivities were strongly dependent on reaction conditions and substrates. Good to excellent regio- and enantioselectivities were obtained with zinc reagents R2Zn and aluminum reagents R3Al. However, the asymmetric conjugate addition of Grignard reagents afforded only moderate to good regio- and enantioselectivities.
DOI : 10.1055/s-0035-1562487
archive ouverte unige:86676
