Publication 38
- Chiral Monodentate Phosphine Ligands for the Enantioselective α− and γ−arylation of Aldehydes
Ivan Franzoni, Laure Guénée, Clément Mazet*
Tetrahedron 2014, 70, 4181-4190
[ Dedicated to Prof. Sarah Reisman on receipt of the Tetrahedron Young Investigator Award 2014 ]
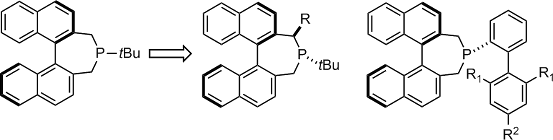
The synthesis of chiral variants of monodentate trialkyl and dialkylbiaryl phosphine ligands elaborated on the binepine scaffold is described. Their application in the Pd-catalyzed intramolecular asymmetric α-arylation of aldehydes and the intermolecular asymmetric γ-arylation of α,β-unsaturated aldehydes provides a mean of validating the design of these ligands. For the first reaction, excellent reactivities have been obtained while only modest enantioselectivities were measured. Aside from enantioselectivity, the second reaction offers additional challenges associated with intramolecularity and regioselectivity. With the formal chiral trialkyl monodentate phosphine ligands, good yield, high olefin stereocontrol and perfect γ-selectivity were obtained while the enantioselectivity remained in the low but promising range.
archive ouverte unige:36857
DOI de la version éditeur : 10.1016/j.tet.2014.02.079
