Publication 52
- Direct Access to Furoindolines by Palladium-Catalyzed Intermolecular Carboamination
Vincent Bizet, Gustavo M. Borrajo-Calleja, Celine Besnard, Clément Mazet*
ACS Catal. 2016, 6, 7183-7187
[ Highlighted in: Synfacts 2016, 1257 ]
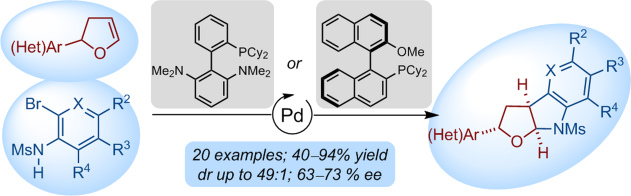
A versatile Pd-catalyzed intermolecular syn-carboamination of dihydrofurans giving access to the ubiquitous furoindoline motif is described. The efficiency of the process relies on the use of Buchwald-type biarylphosphines and the perfect control for site-selectivity of Pd insertion across the C=C bond. A catalytic sequence consisting of Heck and carboamination cross-coupling reactions from readily available dihydrofurans affords - in usually high chemical yields and highlevels of diastereocontrol - poly(hetero)cyclic compounds that would be difficult to access by established methods. Encouraging preliminary results for the enantioselective carboamination of 2,3-dihydrofurans are also disclosed.
archive ouverte unige:89512
DOI de la version éditeur : 10.1021/acscatal.6b02238
