Publication 55
- Exploring Site Selectivity of Iridium Hydride Insertion into Allylic Alcohols: Serendipitous Discovery and Comparative Study of an Organic and an Organometallic Catalysts for the Vinylogous Peterson Elimination
Houhua Li, Daniele Fiorito, Clément Mazet*
ACS Catal. 2017, 7, 1554-1562
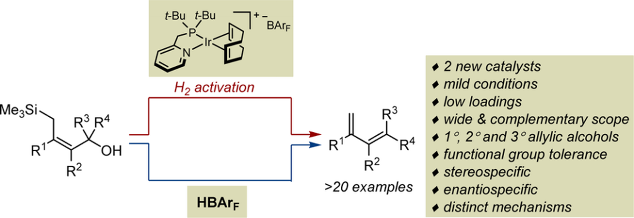
The vinylogous Peterson elimination of a broad range of primary, secondary and tertiary silylated allylic alcohols by two distinct and complementary catalytic systems - a cationic iridium complex and a Brønsted acid - is reported. These results are unexpected. Non-silylated substrates are typically isomerized into aldehydes and silylated allylic alcohols into homoallylic alcohols with structurally related iridium complexes. Although several organic acids and bases are known to promote the vinylogous Peterson elimination, the practicality, mildness, functional group tolerance and generality of both catalysts are simply unprecedented. Highly substituted C=C bonds, stereochemically complex scaffolds, vicinal tertiary and quaternary (stereo)centers are also compatible with the two methods. Both systems are stereospecific and enantiospecific. After optimization, a vast number of dienes with substitution patterns that would be difficult to generate by established strategies are readily accessible. Importantly, control experiments secured that traces of acid that may be generated upon decomposition of the in situ generated iridium hydride are not responsible for the activity observed with the organometallic species. Upon inspection of the reaction scope and on the basis of preliminary investigations, a mechanism involving iridium-hydride and iridium-allyl intermediates is proposed to account for the elimination reaction. Overall, this study confirms that site selectivity for [Ir−H] insertion across the C=C bond of allylic alcohols is a key parameter for the reaction outcome.
archive ouverte unige:95401
DOI de la version éditeur : 10.1021/acscatal.6b03376
